Abstract
To investigate a possible common environmental exposure that may partially explain the observed decrease in human semen quality, we correlated seminal plasma and blood cadmium levels with sperm concentration and sperm motility. We studied three separate human populations: group 1, infertility patients (Long Island, NY, USA); group 2, artificial insemination donors (AID) (Rochester, NY, USA); and group 3, general population volunteers (Rochester, NY, USA). Information about confounding factors was collected by questionnaire. Seminal plasma cadmium did not correlate with blood cadmium (Spearman correlation, n = 91, r = −0.092, P = 0.386, NS). Both blood and seminal plasma cadmium were significantly higher among infertility patients than the other subjects studied (for example, median seminal plasma cadmium was 0.282 µg/L in infertility patients versus 0.091 µg/L in AID and 0.092 µg/L in general population volunteers; Kruskal-Wallis test, P < 0.001). The percentage of motile sperm and sperm concentration correlated inversely with seminal plasma cadmium among the infertility patients (r = −0.201, P < 0.036 and r = −189, P < 0.05, respectively), but not in the other two groups. Age (among infertility patients) was the only positive confounder correlating with seminal plasma cadmium. To validate our human findings in an animal model, we chronically exposed adolescent male Wistar rats to low-moderate cadmium in drinking water. Though otherwise healthy, the rats exhibited decreases in epididymal sperm count and sperm motility associated with cadmium dose and time of exposure. Our human and rat study results are consistent with the hypothesis that environmental cadmium exposures may contribute significantly to reduced human male sperm concentration and sperm motility.
Similar content being viewed by others
Introduction
Human fecundity appears to be on the decline (1,2), a situation that cannot be attributed solely to an increase in contraception. Rather, a body of data suggests that poor semen quality is markedly increasing and is likely to be a contributing factor (1,2).
Several studies suggest that sperm concentration has decreased over time (3–6). Although there are some conflicting findings (7,8), three related factors may explain sperm concentration decline. First, because spermatogenesis is testosterone dependent (9–11), a parallel decrease in serum testosterone can be expected, and a preliminary report suggests that this has occurred in American men (12). Second, because time to pregnancy increases with decreasing sperm concentrations (13), time to pregnancy can be expected to increase, and this has been reported (14). Third, the demand for in vitro fertilization (IVF)/intracytoplasmic sperm injection continues to increase, a situation that has been suggested to indicate a decline in male fertility potential (15). The basic question remains, what causes these changes to occur? One possibility is environmental toxicant exposures.
The male appears more susceptible than the female to the effects of occupational or environmental exposures to reproductive toxicants (16–19), and it is not surprising that environmental agents have been postulated to be contributory to deteriorating semen quality and a decline in male reproductive health (1,20,21).
Studies of the effects of environmental agents on semen quality in wildlife (22–25) and in man (1,20,21) have largely focused on organic toxicants with potential endocrine disrupting activity, but heavy and transition metal endocrine disruptors, such as cadmium, may be influential as well.
Cadmium has been recognized as an endocrine disruptor because of adverse effects on wildlife reproduction (26–28), disruption of steroidogenesis and spermatogenesis in laboratory animals (29–31), and ability to bind to androgen and estrogen receptors (32). Results of several studies suggest that the testis may be exquisitely sensitive to cadmium, with cadmium exposure leading to profound testicular damage and irreversible infertility (33–35) without affecting any other organ system. In addition, cadmium preferentially accumulates in both human and animal reproductive organs (36,37).
Our interest in the reproductive toxicity of cadmium developed from observations that cadmium was elevated in the seminal plasma of infertile men with varicocele (38) as well as in the testes of oligo- and oligoasthenozoospermic men with or without varicoceles (39–41). These men were not occupationally exposed to cadmium, and the majority of them were also nonsmokers. This eliminates one major source of human cadmium exposures, active or passive cigarette smoking. Elevated seminal plasma and testicular cadmium in these subjects could be derived from contaminated food, another major source of environmental cadmium. Other potential sources of environmental exposure include drinking water, cosmetics, herbal remedies, and industrial pollution (42,43). Interpretations of reported data are complicated by the presence of a number of other toxicants. Thus, the relationship between cadmium and human male infertility has been a matter of some debate (42), and the cadmium levels found in the general population indicate potential for additional study. Thus, we investigated the status of cadmium in infertile men and the general population.
Study populations in investigations of cadmium levels have been generally limited to men seeking treatment for infertility (44), and despite protestations to the contrary, the results may not be representative of the general population. Furthermore, studies of population levels of cadmium have been limited and are relatively old, and in these studies cadmium exposures were based on blood cadmium levels. In addition, these studies also suffer from a variety of limitations, including the lack of standardized definitions for “fertility” and “infertility” (42).
Other studies provide some evidence suggesting that geographic variations (and thus environmental exposures) contribute to blood cadmium levels (45–52), along with industrial exposure, diet, and ethnicity (48,49). In contrast, seasonal variation appeared negligible (50).
Blood cadmium concentrations seem to reflect primarily current exposure (46,48,53–56). Cumulative exposures may be more important for reproductive toxicology, because reproductive tract cadmium levels increase over time, and elevations in cadmium may not become apparent until humans are older than 40 years (37). Specific studies suggest that seminal plasma cadmium levels seem representative of cumulative exposures (56) and that cadmium concentrations are higher in seminal vesicles than in any other compartment of the male reproductive tract (37).
Therefore, we undertook the current study to evaluate two questions: whether cadmium levels in seminal plasma differ among infertility patients, men who were participating as donors in an artificial insemination program, and men in the general population; and whether cadmium levels in seminal plasma affect normal semen parameters and clinical outcomes. To support these human findings, we also obtained data from Wistar rats fed cadmium in their drinking water to study the effect of this toxic metal on spermatogenesis and sperm motility.
Materials and Methods
Chemicals
Concentrated hydrochloric and nitric acids (Optima grade = trace metal ion free) were purchased from Fisher Scientific (Pittsburgh, PA, USA). Cadmium chloride was ordered from Alfa Aesar (Johnson Matthey Chemicals, London, UK) (Cat. No. 12373 ACS; CdCl2.2.5H2O; 1 gram of cadmium equals 2.03 grams of 81% pure CdCl2). All other chemicals, unless otherwise specified, were reagent grade or higher (Sigma, St. Louis, MO, USA).
Human Blood and Semen Specimens
Institutional review board approval. Subjects were recruited from two geographic locations. All protocols employing human subjects were reviewed and approved by the institutional review boards of North Shore University Hospital and the University of Rochester Medical Center. Blood and semen were collected from three distinct populations: group 1, infertility patients (n = 140); group 2, artificial insemination (AI) donors (n = 15); and group 3, general population volunteers (n = 36). Subjects with antisperm antibodies (as determined by the protocol of Bronson et al. [57]) were excluded from this study.
Group 1: infertility patients. One blood and two semen specimens were obtained from male partners of couples undergoing their first cycle of in vitro fertilization (n = 140) during the period February 1995 to August 1996. This population was based in the Long Island, NY, area and has been previously studied (38,58–61). Cadmium values for seminal plasma were averaged across each subject before examination of the relationship between mean cadmium and other parameters studied.
Insemination policy was based on sperm concentration and motility according to World Health Organization (62) criteria and by acrosome morphology (58). Dose-compensated IVF inseminations were performed: the number of sperm used for each insemination was adjusted based on sperm number and acrosome morphology such that each oocyte was exposed to ≥25,000 sperm with normal acrosomes/mL (58).
Only portions of coded specimens produced for diagnostic purposes by these subjects 2 to 4 wks before the IVF cycle were studied and were obtained at the point of discard. Informed consent was not required for these unidentified specimens.
Group 2: AI donors. Men enrolled as semen donors in an AID program (n = 15) at the University of Rochester Medical Center participated after giving written informed consent. Each subject provided 2 to 3 blood and 3 to 10 semen specimens during the period October 1998 through June 2000. Cadmium values for blood and seminal plasma were averaged across each subject (and in four cases also by charybdotoxin phenotype; 63) before examination of the relationship between mean cadmium and other parameters studied. This population was based in the greater Rochester, NY, area and has been previously studied (63).
All men were qualified for semen donation according to standards established by the New York State Department of Health. All semen specimens were quarantined for 6 months prior to use. Pregnancy rates were obtained using established laboratory methods (64).
Group 3: general population volunteers. Unselected males answering an advertisement for research participation (n = 35) were recruited into the study after giving written informed consent. Each subject provided 1 or 2 blood samples and two to six semen specimens during the period October 1998 through June 2000. Cadmium values for blood and seminal plasma were averaged across each subject before examination of the relationship between mean cadmium and other parameters studied. This population was based in the greater Rochester, NY, area and has not been previously studied.
Semen analysis. In all three groups of subjects, fresh semen specimens were collected by masturbation. Subjects in group 1 collected semen after 2 to 3 d of abstinence from ejaculation. Subjects in groups 2 and 3 collected semen after 3 d of abstinence. All semen specimens were allowed to liquefy before analysis.
For subjects in group 1, sperm concentration and motility in whole semen were determined by microscopic evaluation (62). Morphology assessment of fixed, unstained sperm included evaluation of sperm head size and shape and acrosome size (58).
For subjects from the other two populations, sperm concentration and morphology in whole semen (after air drying and staining with Stat III stain; MidAtlantic Diagnostics, Mount Laurel, NJ, USA) were determined by microscope evaluation (65), and sperm motility was assessed by computer-assisted sperm analysis (IVOS analyzer; Hamilton-Thorne, Beverly, MA, USA) as previously described (63). After removal of an aliquot for preparation of seminal plasma (63), the remaining semen was cryopreserved by use of standard laboratory protocols (63).
Preparation of Blood Plasma and Seminal Plasma for Experimental Analysis
Venous blood samples were obtained from study participants using routine phlebotomy procedures. All venous blood samples were taken between 8:30 and 10:00 AM. Approximately 10 mL of blood was drawn into small purple-top vacutainer tubes. Following clotting, the blood tube was centrifuged at 2500–3000g for 5 min. The plasma supernatant was placed into a sterile acid-washed, metal ion-free microfuge tube (59,63), and stored frozen at −80°C until assay. For analysis of serum hormone levels, residual hemoglobin was removed from 1 mL of serum by filtration through a 10-kD cutoff microfuge filter unit (Millipore, Lakeland, FL, USA) prior to freezing.
Semen was collected between 6:30 and 9:00 AM and processed within 60 min of collection. All semen was collected into metal ion-free sterile containers. Seminal plasma was prepared by use of standard laboratory protocols (63) and stored frozen at −80°C until assay.
Determination of Cadmium Levels in Blood Plasma and Seminal Plasma
Stringent efforts were made to exclude exogenous metal exposures during sampling, sample processing, and analysis, and the methods used have been previously described (59).
Cadmium levels were determined by using standard laboratory protocols (38,61). In brief, specimens were acid digested under high pressure in a microwave and then assayed for cadmium on a SpectrAA 250 Plus atomic absorption spectrometer equipped with a GTA 97 graphite furnace (Varian Instruments, Walnut Creek, CA, USA). We used a calibration curve prepared from a serially diluted cadmium standard (Inorganic Ventures, Lakewood, NJ, USA). Each specimen was assayed in triplicate for cadmium content. As previously reported (59), there was <5% intraspecimen variation between cadmium determinations.
Sperm Function Testing
In group 1, motile sperm populations were isolated from fresh semen by use of swim-up. In group 2 and group 3, motile sperm populations were isolated from thawed semen by using a three-step Percoll density gradient as previously described (66). One-half of each preparation was assayed for biomarker expression immediately after isolation and the other half after overnight capacitation performed according to standard laboratory protocols (63,66).
The increase in mannose receptor expression after capacitation was determined by binding of fluorescein isothiocyanate-conjugated mannosylated bovine serum albumin to the sperm head (66). The increase in premature (“spontaneous”) acrosome loss upon capacitation (60) and the ability of capacitated sperm to undergo acrosome loss induced by exposure of sperm to model zona ligands containing mannose (67) or to 1 µg/mL progesterone (68,69) were assessed by labeling of acrosome content with rhodamine-labeled Pisum sativum agglutinin (70). Labeled sperm were viewed at 600× magnification with an Olympus BX-50 UV-epifluorescence microscope (Olympus, Lake Success, NY, USA).
Note that expression of all biomarkers tested correlates with the rate of fertilization in IVF (58,60,67,69).
Potentially Confounding Variables
Subject age, the presence or absence of varicoceles, lifestyle variables (subject occupations, cigarette smoking, consumption of beverages containing alcohol or caffeine, and/or use of prescription medications or vitamins/mineral supplements) were assessed by questionnaire administered by the intake nurse or the technician who drew the blood samples (59,63).
Animal Model
Institutional animal care and use committee approval. The protocols employed to develop an animal model to study the reproductive toxic effects of cadmium were approved by the institutional animal care and use committee of the North Shore-Long Island Jewish Health System. Animals were maintained in accordance with standards set forth in the Animal Welfare Act.
Cadmium solutions. Oral hydration solutions containing 14% sucrose alone or 14% sucrose supplemented with environmentally realistic low doses of cadmium (5 mg/L, 50 mg/L, and 100 mg/L) were prepared as previously described (43). Cadmium levels were verified by atomic absorption prior to animal administration (43).
Exposure of rats to cadmium. Five-wk-old (pubertal) male Wistar Hanover rats (Charles River Laboratories, Wilmington, MA, USA) were given ad libitum access to a nutritionally complete solid diet in combination with deionized water containing 14% sucrose or 14% sucrose supplemented with cadmium as described above. Animal weight and water consumption (by weight over a 24-h period) were determined twice a wk (43). The animals examined in relation to our three human populations were killed after 4 wk and 8 wk of cadmium exposure (43).
At the time of death we determined testicular cadmium levels by using atomic absorption spectroscopy with methods previously described (40) and similar to those outlined above for seminal plasma. Testes accumulated cadmium based on time and dose (43).
Determination of epididymal sperm count and motility. At the time of death we weighed dissected cauda epididymes and then minced them to release sperm. The total number of sperm in each epididymis (sperm count) was determined by counting sperm on a hemacytometer, and motility was determined as previously described (43).
Statistical Methods
We performed statistical analyses using the SAS version 9.1.3 software package (SAS Institute, Cary, NC, USA) and the SigmaStat v 3.0 software package (SSPS, Chicago, IL, USA). Statistical significance was set at P < 0.05.
Analysis of the correlation between continuous variables (for example, seminal plasma cadmium level) with semen parameters, serum hormone levels, and clinical outcomes was performed with the Spearman correlation coefficient, a nonparametric counterpart to the Pearson correlation coefficient.
The comparison of cadmium levels among the three populations studied and among group 1 subjects with differing pregnancy outcomes was performed by using the Kruskal-Wallis test, a nonparametric counterpart to the ANOVA test.
The relationship between a categorical variable, such as pregnancy (yes/no) or cigarette smoking (currently or ever) (yes/no), and a continuous variable (for example, seminal plasma cadmium level) was assessed by use of the Mann-Whitney test, a nonparametric counterpart to the two-sample t test.
The effect of oral, low-dose, chronic cadmium exposures on Wistar rat cauda epididymal sperm count and motility was examined using ANOVA, with post hoc multiple comparisons performed with the Holm-Sidak (H-S) method.
Results
Group 1: Infertility Patients
Analysis of seminal plasma cadmium levels as a predictor of IVF fertilization rates was part of a larger prospective study that examined parameters potentially affecting IVF outcome (58–61,67).
Cadmium in blood plasma and seminal plasma. Of the 140 subjects in group 1, 91 subjects were assessed for blood plasma cadmium levels and 132 for cadmium in seminal plasma. Cadmium levels in blood and seminal plasma varied over a relatively wide range (Table 1; 59). No relationship was detected between blood cadmium levels and seminal plasma cadmium levels (Table 2).
Cadmium levels and semen parameters. Of the 118 group 1 subjects for whom semen parameters were available, 34 (28.8%) had normal semen parameters and 84 (71.2%) were defined as male factor according to World Health Organization (62) criteria and acrosome morphology (58). Among the male factor cases, 44 had one semen parameter defect (oligozoospermia, asthenozoopsermia, or teratozoospermia), 24 had defects in two semen parameters, and 16 presented with defects in all three semen parameters.
In group 1, seminal plasma cadmium levels were negatively correlated with sperm concentration and sperm motility in whole semen (Table 3). This relationship was not strengthened when the relationship between semen parameters and cadmium was examined in the subsets of men with various abnormal semen parameters (not shown).
In contrast, blood plasma cadmium levels were unrelated to semen parameters (Spearman correlations, n = 80; sperm concentration, r = −0.017, P = 0.877; normal morphology, r = −0.059, P = .604; motility, r = −0.051, P = 0.654; all not significant).
Cadmium levels and serum hormone concentrations. In group 1 subjects, seminal plasma cadmium levels were unrelated to circulating levels of follicle-stimulating hormone, luteinizing hormone, or testosterone (Table 3). Similar findings were obtained for blood plasma cadmium levels (Spearman correlations, n = 24: follicle-stimulating hormone, r = −0.036, P = 0.865; luteinizing hormone, r = −0.122, P = 0.569; testosterone, r = 0.041, P = 0.848; all not significant).
Cadmium levels and sperm bio-marker expression. Seminal plasma cadmium levels in group 1 subjects had no association with human sperm biomarker expression (Table 3). This finding was consistent with our previous findings about cadmium (61). Likewise, blood plasma cadmium levels were unrelated to biomarker expression (Spearman correlations: mannose receptor expression, n = 75, r = 0.138, P = 0.238; spontaneous acrosome loss, n = 66, r = 0.023, P = 0.857; mannose-induced acrosome loss, n = 52, r = −0.162, P = 0.252; and progesterone-induced acrosome loss, n = 17, r = 0.130, P = 0.617; all not significant).
Cadmium levels and IVF fertilization and pregnancy rates. No association was detected between sperm concentration and IVF fertilization rates (Table 4). In contrast, sperm motility in whole semen was positively correlated with fertilization rates.
No association was detected between seminal plasma cadmium levels and IVF fertilization rates (Table 5A). Similarly, blood plasma cadmium levels and IVF fertilization rates were unrelated (Spearman correlation, n = 66, r = −0.051, P = 0.686).
Seminal plasma cadmium levels did not differ among those group 1 subjects who did not achieve a pregnancy after embryo transfer, those who achieved a clinical pregnancy only (that is, a quantitative serum human chorionic gonadotrophin assay on d 12 after embryo transfer of ≥5 mIU/mL; 58), and those who had a viable pregnancy (established by a sonogram during wk 5 after embryo transfer; 58) (Table 5B). Blood plasma cadmium levels and pregnancy outcomes were similarly unrelated (not shown; Kruskal-Wallis test, P = 0.365).
Cadmium levels and lifestyle variables. Patients in group 1 ranged in age from 25 to 55 years. Seminal plasma cadmium levels and subject age were positively correlated (Table 6). In contrast, blood cadmium levels were independent of subject age (Spearman correlation, n = 86, r = −0.012, P = 0.914).
Thirty-one subjects in group 1 reported drinking between 0.25 and 4 glasses of an alcoholic beverage per d. Seminal plasma cadmium levels were available for 29 of these subjects and blood plasma cadmium for 17. No relationship was detected between the number of alcoholic beverages consumed per d and seminal plasma (Table 6) or blood plasma (Spearman correlation, r = 0.135, P = 0.598, not significant) cadmium levels. The same findings were obtained when seminal cadmium levels measured in alcohol consumers and nonconsumers (Table 7) or blood plasma cadmium were compared (Mann-Whitney test, consumers [n = 17, median = 0.739, interquartile range = 0.550 to 0.835] versus nonconsumers [n = 63, median = 0.759, interquartile range = 0.616 to 0.803], P = 0.733).
In group 1, 78 subjects reported taking prescription medications. When subjects were categorized by medication usage (yes or no), no difference was observed between users and nonusers with respect to seminal plasma cadmium levels (Table 7) or blood plasma cadmium levels (Mann-Whitney test, users [n = 40, median = 0.744, interquartile range = 0.607 to 0.801] versus nonusers [n = 45, median = 0.759, interquartile range = 0.573 to 0.805], P = 0.650).
Of the 140 group 1 men studied, 98 were taking vitamin and/or mineral supplements. However, comparison of seminal plasma cadmium levels (Table 7) between subjects taking these agents with those who were not revealed no difference. Blood plasma cadmium levels were also unaffected by vitamin and/or mineral supplements (Mann-Whitney test, consumers [n = 9, median = 0.759, interquartile range = 0.629 to 0.801] versus nonconsumers [n = 77, median = 0.750, interquartile range = 0.583 to 0.799], P = 0.592).
Only 16 of the subjects in group 1 smoked cigarettes, and no relationship was detected between the number of cigarettes smoked per d and seminal plasma (Table 6) or blood plasma (Spearman correlation, n = 11, r = 0.132, P = 0.717) cadmium levels. Similarly, seminal plasma cadmium levels of smokers and nonsmokers (Table 7) or blood plasma cadmium (Mann-Whitney test, smokers [n = 11, median = 0.638, interquartile range = 0.425 to 0.785] versus non-smokers [n = 75, median = 0.759, interquartile range = 0.614 to 0.813], P = 0.080) did not differ.
Of the group 1 subjects, 109 reported drinking between 0.5 and 6 beverages containing caffeine per d. Seminal plasma cadmium levels were available from 101 subjects and blood plasma cadmium from 68. Neither seminal plasma (Table 6) nor blood plasma (Spearman correlation, r = −0.032, P = 0.789, not significant) were related to the number of caffeine-containing beverages consumed per d. Consistent with this finding, cadmium levels of those who drank caffeine-containing beverages and those that did not were similar (Table 7). In contrast, blood plasma cadmium differed significantly when compared categorically by caffeine consumption status (yes or no) (Mann-Whitney test, consumers [n = 68, median = 0.746, interquartile range = 0.548 to 0.792] versus nonconsumers [n = 18, median = 0.790, interquartile range = 0.737 to 0.817], P = 0.035).
Unfortunately, no information was captured concerning IVF patients’ occupations and potential exposure to transition and heavy metal ions.
Group 2: AI Donors
Cadmium in blood plasma and seminal plasma. Of the 15 men in group 2, blood and seminal plasma cadmium levels were available from 14 subjects. The range of values and the maximal values for both parameters were lower than those observed in group 1 (Table 1). No relationship was detected between blood and seminal plasma cadmium levels (Table 2).
Cadmium levels and semen parameters. All group 2 men had normal semen parameters according to World Health Organization (65) criteria. Seminal plasma cadmium levels and standard semen parameters were unrelated (Table 3).
Cadmium levels and sperm biomarker expression. At the time of this report, only 2 of the 4 biomarkers have been assessed for group 2—spontaneous acrosome loss and progesterone-stimulated acrosome loss. In contrast to findings for group 1, a positive relationship was detected between seminal plasma cadmium levels and spontaneous acrosome loss in specimens from group 2 subjects (Table 3). Furthermore, when the relationship between seminal plasma cadmium levels and progesterone-stimulated acrosome loss was examined, a Spearman correlation coefficient was obtained (Table 3) that, although not significant, was sufficiently large (r = −0.404) to suggest that a negative relationship might become significant if the sample size were to be increased.
Cadmium levels and pregnancy rates. AID pregnancy rates were available for 12 group 2 subjects. A negative relationship was detected between seminal plasma cadmium concentrations and AID pregnancy rates (Table 5A).
In group 2, pregnancy rates after AID were positively correlated with the percentage of motile sperm (Table 4).
Cadmium levels and lifestyle variables. Subjects in group 2 were between 19 and 31 years old. No relationship was detected between seminal plasma cadmium levels and subject age (Table 6).
In group 2, 7 of the 15 subjects completed the questionnaire concerning lifestyle variables. None smoked cigarettes or were taking prescription medications or vitamins and/or mineral supplements. Four subjects in group 2 reported consuming 0.6 to 1.8 alcoholic beverages, and three reported abstinence from alcohol. Seminal plasma cadmium levels were unrelated to consumption of alcoholic beverages (Tables 6 and 7). Six subjects in group 2 indicated that they drank 0.5 to 2.5 caffeine-containing beverages per d, and one donor did not drink beverages containing caffeine. Seminal plasma cadmium was unrelated to caffeine consumption (Table 6). Only one subject in group 2 reported potential occupational exposure to transition and heavy metals. His seminal plasma cadmium value (0.099 µg/L) was lower than median value for the other six donors (0.102 µg/L).
Group 3: General Population Volunteers
Cadmium in blood plasma and seminal plasma. Seminal and blood plasma samples were obtained from a previously unstudied group of 35 men from the general population. Their cadmium values were indistinguishable from subjects in group 2 (Table 1), and blood and seminal plasma cadmium levels were unrelated (Table 2).
Cadmium levels and semen parameters. The majority of subjects in group 3 had normal semen parameters according to World Health Organization (65) criteria. No relationship was detected between seminal plasma cadmium levels and standard semen parameters (Table 3).
Cadmium levels and sperm bio-marker expression. Sperm from 14 subjects in group 3 were processed for sperm function testing. As with subjects in group 2, this analysis was limited to assessment of spontaneous acrosome loss and the progesterone-stimulated acrosome reaction. Unlike group 2 subjects, in group 3 subjects no relationship was detected between seminal plasma cadmium levels and spontaneous acrosome loss or the ability to undergo a progesterone-stimulated acrosome reaction (Table 3).
Cadmium levels and pregnancy rates. Twenty-two group 3 subjects reported attempting pregnancy by coitus, and 20 of this subgroup achieved live births. Neither semen parameters (Table 4) nor seminal plasma cadmium levels (Table 5A) were predictive of pregnancy by coitus.
Cadmium levels and lifestyle variables. Subjects in group 3 ranged in age from 21 to 44 years (median age 32.5 years [interquartile range: 29 to 36 years]). No relationship was detected between age and seminal plasma cadmium.
All subjects in group 3 completed the questionnaire concerning lifestyle variables. Four subjects in group 3 indicated that they drank alcoholic beverages and three did not. Seminal plasma cadmium and alcohol consumption were unrelated (Tables 6 and 7).
Eight group 3 subjects reported taking prescription medications and 26 did not. No difference was detected between the seminal plasma cadmium levels of those taking these medications and those that did not (Table 7).
No group 3 subjects reported taking vitamin and/or mineral supplements.
Twenty-nine subjects in group 3 reported drinking beverages containing caffeine and one reported abstaining. Seminal plasma cadmium levels were unrelated to consumption of caffeine-containing beverages (Table 6).
Ten subjects in group 3 stated that they smoked cigarettes and 21 stated that they did not smoke. No relationship between seminal plasma cadmium and the number of packs of cigarettes smoked per d was detected (Table 6), and seminal plasma cadmium levels in smokers and nonsmokers did not differ (Table 7).
Nineteen subjects in group 3 were occupationally exposed to transition and heavy metals and 16 were not, but seminal plasma and blood cadmium levels in exposed subjects and nonexposed subjects were similar (Table 8). Pregnancy rates were unaffected by occupation exposure to cadmium (Table 8).
Modeling the Reproductive Toxic Effects of Cadmium in Male Wistar Rats
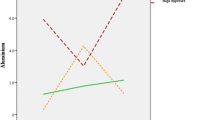
At 4 wks of cadmium exposure, cauda epididymal sperm counts were reduced as cadmium levels were increased (Figure 1). Similar findings were obtained after 8 wks of cadmium exposure (not shown; ANOVA, P < 0.05).
Cadmium exposure results in a time and dose-dependent reduction in sperm count. The control and each cadmium-exposure group (5 mg/L, 50 mg/L, and 100 mg/L) were composed of five Wistar rats. Sperm count was determined individually for the left and right cauda epididymes of each rat and then totaled. Sperm counts (in term of millions of sperm) are presented as means with standard errors. An inverse relationship between cadmium dose and sperm concentration was detected after 4 wks of exposure (ANOVA, P < 0.018). Post hoc pairwise comparisons indicated that sperm counts of control animals and those exposed to 5 mg/L cadmium were indistinguishable (H-S, P = 0.252, not significant). At exposures of 50 mg/L and 100 mg/L, however, there was a dose-dependent decrease in sperm counts as compared with controls (H-S, respectively, P < 0.028 and P < 0.003).
Sperm motility was reduced at 4 wks by cadmium exposure in a dose-dependent manner (Figure 2). At 8 wks of exposure, sperm motility was further decreased and did not differ by dose (Figure 2).
Cadmium in water given ad libitum reduces sperm motility. Sperm motility was determined individually for the left and right cauda epididymes of each rat in the control group (n = 5) and the cadmium-exposed groups (5 mg/L, n = 5; 50 mg/L, n = 5; and 100 mg/L, n = 5). Data are presented as sperm motility of cadmium-treated rats as a percentage of that of control animals. A dose-dependent decrease in sperm motility was observed after exposure to cadmium for 4 wks (ANOVA, P < 0,007; H-S, P < 0,025 to P < 0,001). Sperm motility at 8 wks of cadmium treatment was lower than at 4 wks, and the effect of dose was no longer apparent (ANOVA, P = .239, not significant).
Discussion
Prior studies of the relationship between seminal plasma cadmium and semen parameters have produced conflicting data (review, 42). Seminal plasma cadmium levels have been reported to be unrelated to semen parameters and fertility status (71–74), to be positively correlated with semen parameters (75), to increase as sperm concentration is decreased (76,77), and to be inversely related to semen parameters only in those men whose semen parameters are already adversely compromised (44,78,79). Our results showed an inverse correlation of seminal plasma cadmium levels with semen parameters in men from infertile couples (group 1) but not known fertile males (group 2) or volunteers from the general population (group 3). This finding is consistent with two of the above studies (76,77) but not others. We recognize that the infertility population (group 1) included a larger number of subjects and a greater range of cadmium concentrations and thus provided more power in detecting associations than the other two populations studied. Nevertheless, our data suggest that seminal cadmium levels are elevated specifically in infertility patients and are associated with decreased semen quality.
To determine if, in fact, cadmium is responsible for poor semen quality, the effects of low-dose, environmentally relevant cadmium exposures were examined in an animal model, the male Wistar rat. Although some rat strains are resistant to the negative testicular effects of cadmium (for example, Sprague Dawley rats; 80), the Wistar rat strain exhibits aberrant testicular histology and infertility after chronic low-dose exposure to cadmium (81). In our study, chronic exposure to environmentally relevant cadmium resulted in dose- and time-dependent decreases in sperm count and sperm motility. These data are consistent with prior findings of the effects of chronic, low-dose exposure to cadmium in drinking water in Wistar rats (82–84) and mimicked our findings in group 1 infertility patients. Thus, although our study conclusions require confirmation by studies of larger human populations, these data suggest that a causal relationship exists between elevated reproductive tract cadmium levels and poor semen quality in susceptible individuals. The concept of susceptibility will be discussed further below.
We did not find evidence for cadmium action as an endocrine disruptor in this study. This finding was unexpected, because Martin and colleagues presented convincing evidence that cadmium can induce estrogenic activities in cell cultures (85,86) and ovariectomized animals (87), and estrogen plays an important role in regulating the adult male reproductive tract (review, 88). However, testicular testosterone levels were not assessed in this study. Testicular testosterone levels are one-hundred-fold higher than normal serum testosterone levels (89), and these high levels are required to support spermatogenesis (90). It is possible that testicular testosterone levels are more sensitive to the effects of cadmium than are serum levels. Thus, in the absence of testicular testosterone measurements, our findings are consistent with a prior report that cadmium can impair semen quality without effects on male reproductive endocrine function (56).
We did not identify an association between cadmium and altered sperm bio-marker function in this study in group 1 (infertility patients) or in group 3 (general population volunteers). This finding is in contrast with our in vivo and in vitro studies of lead and zinc and other metals in seminal plasma (42,59,61,63,91), in which we found both seminal plasma lead and seminal plasma zinc levels to be correlated with expression of sperm functions required for fertilization of oocytes in IVF inseminations, with lead negatively correlated with expression of mannose receptors and progesterone- and mannosestimulated acrosome loss and positively with spontaneous acrosome reactions (59,63; S Benoff, unpublished observations). A negative relationship between seminal plasma lead levels and progesterone-stimulated acrosome loss was also detected in group 3 (general population volunteers) (S Benoff and GM Centola, unpublished observations). Furthermore, these observations are supported by in vitro modeling studies examining the effect of increasing doses of lead on expression of these biomarkers by sperm from known fertile donors (42,59) and in lead-exposed animal models (92,93). Therefore, we attribute the positive relationship between seminal plasma cadmium and spontaneous acrosome loss and the suggested negative relationship between seminal plasma cadmium and progesterone-stimulated acrosome loss detected in group 2 (AI donors) to be artifacts. This is because in group 2 subjects (in contrast to group 1 and group 3 subjects) seminal plasma cadmium and seminal plasma lead levels were positively correlated (94), and because a strong positive relationship between seminal plasma lead and spontaneous acrosome loss and a strong negative relationship between progesterone-stimulated acrosome loss and seminal plasma lead levels have previously been described in group 2 subjects (63). The correlation between seminal plasma cadmium and lead levels has been reported in other human populations (for example, 74). Together, these findings emphasize the need to examine the effect of mixtures of toxicants in future studies (94).
We examined the relationship between seminal plasma cadmium levels and seven potentially confounding variables. First, we examined the effect of increasing age, because Oldereid et al. (37) reported that testicular cadmium levels are age dependent, and elevations in cadmium content are primarily observed after the fourth decade of life. A statistically significant positive relationship between seminal plasma cadmium and patient age was detected in group 1 but not in group 2 or group 3. However, the correlation coefficient in all groups was about 0.2, so that conclusions about the lack of significance in the latter two populations should be tempered owing to the small sample size. Second, we examined the relationship between seminal plasma cadmium and alcohol consumption. Alcohol consumption has alternatively been reported to have no effect on body cadmium burdens (95), to decrease cadmium uptake (96), and to increase cadmium uptake (see for example, 97). The current findings do not help resolve this controversy. Cadmium and alcohol consumption were not associated in any of the three populations studied. Third, we explored whether seminal plasma cadmium levels were influenced by prescription medications. Our rationale was three-fold: (a) chelating agents have been shown to reduce body burden of cadmium (see for example, 98,99), (b) prescription medications may contain metals such as cadmium as a result of contamination of raw materials or through manufacturing equipment (100), and (c) although new methodologies are being considered (101), current methods to detect metal impurities in pharmaceuticals are nonspecific and insensitive (100). Nevertheless, seminal plasma cadmium levels in subjects reporting taking prescription medications did not differ in group 1. Similar findings were obtained for group 3 subjects. Fourth, we investigated whether there was an association between seminal plasma cadmium levels and vitamin and/or mineral supplements. Vitamins can increase urinary secretion of metals such as cadmium or can chelate metals (102,103). Both vitamins and mineral supplements often contain zinc, long known to have a competitive effect in regard to the toxic effects of cadmium in the reproductive tract (see for example, 104). In addition, metal contamination has been detected in dietary supplements (105). We observed, however, that seminal plasma cadmium levels were unrelated to vitamin and/or mineral supplement usage in group 1 or group 3, and none of the subjects in group 2 were taking these supplements. Fifth, we examined the association between cigarette smoking and seminal plasma cadmium levels. A body of literature indicates that cigarette smoking increases both seminal plasma and blood plasma cadmium levels (review, 42), and that cigarette smoking is associated with decreased testis size (106), increased serum reproductive hormone levels (56,106), and decreased sperm concentration (107) and motility (56). However, no group 2 subjects reported smoking cigarettes, and no relationship between seminal plasma cadmium and cigarette smoking was detected in the other two populations. Sixth, because interactions between caffeine and cadmium (108) and caffeine and smoking (109) have been reported, we queried the correlation between seminal plasma cadmium and caffeine consumption. No relationships were detected in any of the three populations participating in this study. Seventh and last, we questioned whether seminal plasma cadmium levels were associated with occupational exposures. Although this association was not examined in group 1, no association was detected between cadmium and occupation in subjects in group 2 or group 3.
Despite the negative relationship between seminal plasma cadmium and sperm concentration and motility in group 1 (infertility patients), no association was detected between cadmium and IVF fertilization rates. Group 1 should be a relatively poor choice to correlate sperm parameters with male fertility (Table 4). Dose-compensated IVF inseminations expose all oocytes to the same number of motile sperm with normal morphology (58). This should suppress sperm concentration and sperm motility contributions to fertility potential that have been reported to occur in the general population (110). In this study, however, motility was positively correlated with fertilization rates in IVF (Table 4). We had previously attributed this relationship to the ability to collect sperm by swim-up (58). However, we recognize that there also may be secondary effects associated with dose-compensated IVF inseminations, particularly as related to substantially increasing the number of sperm with abnormal morphology in the inseminate. In the IVF cycles studied, up to 2 × 106 per mL were employed to inseminate an oocyte, and only 25,000 had normal morphology (58). Morphologically abnormal sperm produce elevated levels of reactive oxygen species (ROS; 111). Elevated ROS production is associated with sperm membrane damage and decreased sperm motility (112), and at least one metaanalysis reveals that IVF fertilization rates are inversely correlated with levels of sperm ROS production (113). Thus, the details of the dose compensation protocol could potentially mask the impact on fertilization rates of toxicants such as cadmium that modulate sperm concentration and motility (Table 5).
In contrast to findings for group 1 subjects, AID pregnancy rates were positively correlated with sperm motility although all group 2 subjects had normal semen parameters. This result is consistent with prior findings (114,115). Sperm concentration is also postulated to be contributory (115–117). Consistent with this hypothesis, although not significant, the correlation coefficient for the relationship between pregnancy and sperm concentration (r = 0.5) suggested that the failure to detect a positive relationship between these parameters was related to small sample size (Table 4). These findings suggest that subjects in group 2 offer a better model system than group 1 in studies of the effect of background (“involuntary” = environmental) toxicant exposures on semen parameters and male fertility potential.
Although semen parameters can predict spontaneous pregnancy (pregnancy by coitus) (110,118), no relationship between semen parameters and pregnancy rates was detected in group 3 subjects (volunteers from the general population). This finding was not completely unexpected because of the small sample size, the overlap in semen parameters between fertile and infertile men (119), and the fact that no data were available regarding female factors, and female factors have a strong impact on pregnancy rates (120).
The results of the current study raise the important question of whether infertility patients are more susceptible to the effects of cadmium on spermatogenesis or semen parameters, a question that has been posed previously (107,121). Currently, only limited information is available about the genetic contribution to sensitivity or resistance to cadmium. Although dietary intake of cadmium is higher in men than women (52), cadmium retention is higher in women than in men; for example, blood, urine, and kidney cadmium are elevated in women compared with men (122–124). In part, elevated retention of cadmium by women can be explained by increased cadmium absorption through the intestinal divalent metal transporter (DMT1) when iron stores are low, such as during menstruation (123), and is supported by observations that differences in blood cadmium between sexes normalizes after menopause (125). However, study of monozygotic versus dizygotic twins demonstrated that 65% of the variance in female blood cadmium concentrations could be attributed to genetic factors (126). In contrast, genetic factors could account for only 13% of the variance in male blood cadmium levels (126). This question leads to the suggestion that mutations in specific genes might contribute to the cadmium burden in the reproductive tract of some infertile men and is supported by studies in animal models (127–132).
Mutations in ion channels are obvious candidates for regulation of testicular sensitivity to cadmium. Cadmium enters cells via ion transporters, including the iron transporter, intestinal divalent metal transporter (mentioned above), and voltage-dependent calcium channels (review, Benoff et al., 43,133,134). Expression of sperm-head voltage-dependent calcium channels containing deletions in exons 7 and/or 8 has been identified in Sertoli and testicular germ cells (135,136) in association with elevated testicular cadmium levels (41,137). Alternate splicing of a sperm-tail voltage-dependent calcium channel in association with elevated testicular cadmium has also been reported (43). However, based on studies of ion transporters in brain, it is likely that the expression of these deleted channels is the result of elevated testicular cadmium, and not the cause (133). In addition, animal studies suggest that the critical transport system is located in the testicular vasculature (see for example, 130).
It is well recognized that cadmium acts on the vasculature, for example, by inducing atherosclerosis (138), hypertension in vivo (139), and vasoconstriction in vitro (140,141). Although the toxic effects of cadmium vary from tissue to tissue, the initial effect of cadmium in all sensitive tissues (liver, kidney, nervous system, ovary, uterus, and placenta), including the testis, is at the level of the vascular endothelium (142). Thus, cadmium entry into the testis first occurs as a breach of the blood-testis barrier (130,141,143). Consistent with these observations, the presence or absence of expression of SLC39A8 (ZIP8; a zinc transporter that is also capable of transporting cadmium) by the testicular vascular endothelium has been associated with sensitivity or resistance to cadmium-induced testicular damage in an animal model (144,145). However, not all studies agree, and other candidate genes have been identified (for example, calcineurin; 146). Examination of the expression of these and other genes in human males in relation to cadmium-induced testicular toxicity will be the subject of future investigations.
Disclosure
We declare that the authors have no competing interests as defined by Molecular Medicine, or other interests that might be perceived to influence the results and discussion reported in this paper.
References
Skakkebaek NE, et al. Is human fecundity declining? Int. J. Androl. 2006;29:2–11.
Jensen TK, et al. Declining trends in conception rates in recent birth cohorts of native Danish women: a possible role of deteriorating male reproductive heath. Int. J. Androl. 2008;31:81–92.
Carlsen E, Giwercman A, Keiding N, Skakkebaek NE. Evidence for decreasing quality of semen during past 50 years. BMJ 1992;305:609–13.
Auger J, Kunstmann JM, Czyglik F, Jouannet P. Decline in semen quality among fertile men in Paris during the past 20 years. N. Engl. J. Med. 1995;332:281–5.
Swan SH, Elkin EP, Fenster L. Have sperm densities declined? A reanalysis of global trend data. Environ. Health Perspect. 1997;105:1228–32.
Swan SH, Elkin EP, Fenster L. The question of declining sperm density revisited: an analysis of 101 studies published 1934–1996. Environ. Health Perspect. 2000;108:961–6.
Saidi JA et al. Declining sperm counts in the United States? A critical review. J. Urol. 1999;161:460–2
Fisch H. Declining worldwide sperm counts: disproving a myth. Urol. Clin. North Am. 2008;35:137–46
Roberts KP, Zirkin BR. Androgen regulation of spermatogenesis in the rat. Ann. N. Y. Acad. Sci. 1991;637:90–106.
O’Donnell L, McLachlan RI, Wreford NG, de Kretser DM, Robertson DM. Testosterone withdrawal promotes stage-specific detachment of round spermatids from the rat seminiferous epithelium. Biol. Reprod. 1996;55:895–901.
Sinha Hikim AP, Swerdloff RS. Hormonal and genetic control of germ cell apoptosis in the testis. Rev. Reprod. 1999;:38–47.
Travison TG, Araujo AB, O’Donnell AB, Kupelian V, McKinlay JB. A population-level decline in serum testosterone levels in American men. J. Clin. Endocrinol. Metab. 2007;92:196–202.
Slama R, et al. Time to pregnancy and semen parameters: a cross-sectional study among fertile couples from four European cities. Hum. Reprod. 2002;17:503–15.
Jensen TK, et al. Poor semen quality may contribute to recent decline in fertility rates. Hum. Reprod. 2002;17:1437–40.
Andersen AN, Erb K. Register data on assisted reproductive technology (ART) in Europe. Including a detailed description of ART in Denmark. Int. J. Androl. 2006;29:12–16.
Welch LS, Schrader SM, Turner TW, Cullen MR.Effects of exposure to ethylene glycol ethers on shipyard painters: II. Male reproduction. Am. J. Ind. Med. 1988;14:509–26.
Staessen J, et al. Effects of exposure to cadmium on calcium metabolism: a population study. Br. J. Ind. Med. 1991;48:710–4.
Spinelli A, Figà-Talamanca I, Osborn J. Time to pregnancy and occupation in a group of Italian women. Int. J. Epidemiol. 1997;26:601–9.
Dickman MD, Leung CK, Leong MK. Hong Kong male subfertility links to mercury in human hair and fish. Sci. Total Environ. 1998;214:165–74.
Toppari J, et al. Male reproductive health and environmental xenoestrogens. Environ. Health Perspect. 1996;104(Suppl 4):741–803.
Skakkebaek NE, Rajpert-De Meyts E, Main KM. Testicular dysgenesis syndrome: an increasingly common developmental disorder with environmental aspects. Hum. Reprod. 2001;16:972–8.
Jobling S, Sumpter JP. Detergent components in sewage effluent are weakly oestrogenic to fish: an in vitro study using rainbow trout (Onchrhynchus mykiss) hepatocytes. Aquatic Toxicol. 1993;27:361–72.
Guilette LJ Jr. Endocrine-disrupting environmental contaminants and reproduction: lessons from the study of wildlife. In: Popking DR, Peddle LJ, eds. Women’s health today: perspectives on current research and clinical practice. New York: Parthenon Publication Group; 1994. p 201–7.
Guillette LJ Jr, Edwards TM, Moore BC. Alligators, contaminants and steroid hormones. Environ. Sci. 2007;14:331–47.
Guillette LJ Jr, Edwards TM. Environmental influences on fertility: can we learn lessons from studies of wildlife? Fertil. Steril. 2008;89(2 Suppl): e21–4.
Vodela JK, Lenz SD, Renden JA, McElhenney WH, Kemppainen BW. Drinking water contaminants (arsenic, cadmium, lead, benzene, and trichloroethylene), 2: effects on reproductive performance, egg quality, and embryo toxicity in broiler breeders. Poult. Sci. 1997;76:1493–500
Zacharewski T. Identification and assessment of endocrine disruptors: limitations of in vivo and in vitro assays. Environ. Health Perspect. 1998; 106(Suppl 2):577–82.
Migliarini B, et al. Effects of cadmium exposure on testis apoptosis in the marine teleost Gobius niger. Gen. Comp. Endocrinol. 2005;142:241–7.
Nordberg GF. Effects of long-term cadmium exposure on the seminal vesicles of mice. J. Reprod. Fertil. 1975;45:165–7.
Sen Gupta R, et al. Effect of ascorbic acid supplementation on testicular steroidogenesis and germ cell death in cadmium-treated male rats. Mol. Cell. Endocrinol. 2004;221:57–66.
Thompson J, Bannigan J. Cadmium: toxic effects on the reproductive system and the embryo. Reprod. Toxicol. 2008;25:304–15.
Takiguchi M, Yoshihara S. New aspects of cadmium as endocrine disruptor. Environ. Sci. 2006;13:107–16.
Alsberg CI, Schwartze EW. Pharmacological action of cadmium. J. Pharmacol. 1919;20:13:504–5.
Parizek J, Zahor Z. Effect of cadmium salts on testicular tissue. Nature 1956;177:1036–7.
Parizek J. The destructive effect of cadmium ion on testicular tissue and its prevention by zinc. J. Endocrinol. 1957;15:56–63.
Danielsson BRG, Dencker L, Lindgren A, Tjalve H. Accumulation of toxic metals in male reproductive organs. Arch. Toxicol. 1984; (Suppl 7): 177–80.
Oldereid NB, Thomassen Y, Attramadal A, Olaisen B, Purvis K. Concentrations of lead, cadmium and zinc in the tissues of reproductive organs of men. J. Reprod. Fert. 1993;99:421–5.
Benoff S, et al. A potential role for cadmium in the etiology of varicocele-associated infertility. Fertil. Steril. 1997;67:336–47.
Benoff S, Gilbert BR. Varicocele and male infertility, part I: preface. Hum. Reprod. Update 2001;7:47–54.
Benoff S, Millan C, Hurley IR, Napolitano B, Marmar JL. Bilateral increased apoptosis and bilateral accumulation of cadmium in infertile men with left varicocele. Hum. Reprod. 2004;19:616–27.
Marmar JL, Benoff S. The safety of ultrasonically guided testis aspiration biopsies and efficacy of use to predict varicocelectomy outcome Hum. Reprod. 2005;20:2279–88.
Benoff S, Jacob A, Hurley IR. Male infertility and environmental exposure to lead and cadmium. Hum. Reprod. Update 2000;6:107–21.
Benoff S, Auborn K, Marmar JL, Hurley IR. Link between low-dose environmentally relevant cadmium exposures and asthenozoospermia in a rat model. Fertil. Steril. 2008;89(Suppl 1):e73–9.
Pant N, et al. Lead and cadmium concentration in the seminal plasma of men in the general population: Correlation with sperm quality. Reprod. Toxicol. 2003;17:447–50.
Elinder CG, et al. Cadmium exposure from smoking cigarettes: variations with time and country purchased. Environ. Res. 1983;32:220–7.
Friberg L, Vahter M. Assessment of exposure to lead and cadmium through biological monitoring: results of a UNEP/WHO global study. Environ. Res. 1983;30:95–128.
Moreau T, et al. Blood cadmium levels in the general male population with special reference to smoking. Arch. Environ. Health 1983;38:163–7.
Pocock SJ, Delves HT, Ashby D, Shaper AG, Clayton BE. Blood cadmium concentrations in the general population of British middle-aged men. Hum. Toxicol. 1988;7:95–103.
Chia S-E, Chan O-Y, Sam C-T, Heng B-H. Blood cadmium levels in non-occupationally exposed adult subjects in Singapore. Sci. Total Environ. 1994;145:119–23.
Watanabe T, Koizumi A, Fujita H, Kumai M, Ikeda M. Cadmium levels in the blood of inhabitants in nonpolluted areas in Japan with special references to aging and smoking. Environ. Res. 1983;31:472–83.
Watanabe T, Kasahara M, Nakatsuka H, Ikeda M. Cadmium and lead contents of cigarettes produced in various areas of the world. Sci. Total Environ. 1987;66:29–37.
Watanabe T, et al. Gender-related difference, geographic variation and time trend in dietary cadmium intake in Japan. Sci. Total Environ. 2004;329:17–27.
Elinder CG, Friberg L, Lind B, Jawaid M. Lead and cadmium levels in blood samples from the general population of Sweden. Environ. Res. 1983;30:233–53.
Jarup L, Rogenfelt A, Elinder CG, Nogawa K, Kjellstrom T. Biological half-time of Cd in the blood of workers after cessation of exposure. Scand. J. Work Environ. Health 1983;9:327–331.
Sartor FA, et al. Impact of environmental cadmium pollution on cadmium exposure and body burden. Arch. Environ. Health 1992;47:347–53.
Telisman S, et al. Semen quality and reproductive endocrine function in relation to biomarkers of lead, cadmium, zinc and copper in men. Environ. Health Perspect. 2000;108:45–53.
Bronson RA, Cooper GW, Rosenfeld DL. Correlation between regional specificity of antisperm antibodies to the spermatozoan surface and complement mediated sperm immobilization. Am. J. Reprod. Immunol. 1982;2:222–4.
Benoff S, et al. Numerical dose-compensated in vitro fertilization inseminations yield high fertilization and pregnancy rates. Fertil. Steril. 1999;71:1019–28.
Benoff S, et al. Increased seminal plasma lead levels adversely affect the fertility potential of sperm in in-vitro fertilization. Hum. Reprod. 2003;18:374–383.
Hershlag A, et al. Acrobeads test as a predictor of fertilization in vitro. Am. J. Reprod. Immunol. 1997;37:291–9.
Hurley IR, Hershlag A, Benoff S. Mannose receptors, zinc and fertilization success. Arch. Sexually Transmit. Dis. HIV 1997;11:241–53.
World Health Organization. (1992) Collection and examination of human semen. In: WHO laboratory manual for the examination of human semen and human sperm-cervical mucus interaction. 3rd ed. Cambridge University Press, New York, pp. 3–26.
Benoff S, Hurley IR, Millan C, Napolitano B, Centola GM. Seminal lead concentrations negatively affect outcomes of artificial insemination. Fertil. Steril. 2003;80:517–25.
Centola GM, Herko R, Andolina E, Abbott S. Tracking of insemination outcomes: a novel approach. Fertil. Steril. 2000;74:607–8.
World Health Organization. (1999) Collection and examination of human semen. In: WHO laboratory manual for the examination of human semen and human sperm-cervical mucus interaction. 4th ed. Cambridge University Press, New York, pp 4–27.
Benoff S, et al. Human sperm fertilizing potential in vitro is correlated with differential expression of a head-specific mannose-ligand receptor. Fertil. Steril. 1993;59:854–62.
Benoff S, et al. Use of mannose ligands in IVF screens to mimic zona pellucida-induced acrosome reactions and predict fertilization success. Mol. Hum. Reprod. 1997;3:839–46.
Benoff S, et al. Co-expression of mannose-ligand and non-nuclear progesterone receptors identifies an acrosome reaction-inducible subpopulation in fertile donor sperm. Am. J. Reprod. Immunol. 1995;34:100–15.
Jacob A et al. Human sperm non-nuclear progesterone receptor expression is a novel marker for fertilization outcome. Mol. Hum. Reprod.. 1998;4:533–42.
Cross NL, Morales P, Overstreet JW, Hanson FW. Two simple methods for detecting acrosomereacted human sperm. Gamete Res. 1986;15:213–26.
Keck C, et al. Lack of correlation between cadmium in seminal plasma and fertility status of nonexposed individuals and two cadmium-exposed patients. Reprod. Toxicol. 1995;9:35–40.
Hovatta O, et al. Aluminum, lead and cadmium concentrations in seminal plasma and spermatozoa, and semen quality in Finnish men. Hum. Reprod. 1998;13:115–9.
Dawson EB, Ritter S, Harris WA, Evans DR, Powell LC. Comparison of sperm viability with seminal plasma metal levels. Biol. Trace Elem. Res. 1998;64:215–9.
Kasperczyk A, et al. Lead and cadmium concentration in human semen [in Polish]. Ginekol. Pol. 2002;73:449–53.
Noack-Fuller G, De Beer C, Seibert H. Cadmium, lead, selenium, and zinc in semen of occupationally exposed men. Andrologia 1993;25:7–12.
Xu DX, et al. The associations among semen quality, oxidative DNA damage in human spermatozoa and concentrations of cadmium, lead and selenium in seminal plasma. Mutat. Res. 2003;534:155–63.
Wu HM, et al. Cadmium level in seminal plasma may affect pregnancy rate for patients undergoing infertility evaluation and treatment. Reprod. Toxicol. 2008;25:481–4.
Xu B. Chia SE, Tsakok M, Ong CN. Trace elements in blood and seminal plasma and their relationship to sperm quality. Reprod. Toxicol. 1993;7:613–8
Omu AE, Dashti H, Mohame. AT, Mattappallil AB. Significance of trace elements in seminal plasma of infertile men. Nutrition 1995;11:502–5.
Griffin JL, Walker LA, Shore RF, Nicolson JK. Metabolic profiling of chronic cadmium exposure in the rat. Chem. Res. Toxicol. 2001;14:1428–34.
Saygi S, Deniz G, Kutsal O, Vural N. Chronic effects of cadmium on kidney, liver, testis, and fertility of male rats. Biol. Trace Elem. Res. 1991;31:209–14.
Laskey JW, Rehnberg GL, Favor MJ, Cahill DF, Pietrzak-Flis Z. Chronic ingestion of cadmium and/or tritium, II: effects on growth, development, and reproductive function. Environ. Res. 1980;22:466–75.
El-Demerdash FM, Yousef MI, Kedwany FS, Baghdadi HH. Cadmium-induced changes in lipid peroxidation, blood hematology, biochemical parameters and semen quality in male rats: protective role of vitamin E and beta-carotene. Food Chem. Toxicol. 2004;42:1563–71.
Amara S, et al. Preventive effect of zinc against cadmium-induced oxidative stress in the rat testis. J. Reprod. Dev. 2008;54:129–34.
Garcia-Morales P, et al. Effect of cadmium on estrogen receptor levels and estrogen-induced responses in human breast cancer cells. J. Biol. Chem. 1994;269:16896–901.
Stoica A, Katzenellenbogen BS, Martin MB. Activation of estrogen receptor-alpha by the heavy metal cadmium. Mol. Endocrinol. 2000;14:545–53.
Johnson MD, et al. Cadmium mimics the in vivo effects of estrogen in the uterus and mammary gland. Nature Med. 2003;9:1981–4.
Hess RA. Estrogen in the adult male reproductive tract: a review. Reprod. Biol. Endocrinol. 2003;1:52.
Jarow JP, Chen H, Rosner TW, Trentacoste S, Zirkin BR. Assessment of the androgen environment within the human testis: minimally invasive method to obtain intratesticular fluid. J. Androl. 2001;22:640–5
Coviello AD, et al. Intratesticular testosterone concentrations comparable with serum levels are not sufficient to maintain normal sperm production in men receiving a hormonal contraceptive regimen. J. Androl. 2004;25:931–8.
Benoff S, et al. Metals ions and human sperm mannose receptors. Andrologia 2000;32:317–29.
Johansson L. Premature acrosome reaction in spermatozoa from lead-exposed mice. Toxicology 1989;54:151–62.
Hsu PC, Hsu CC, Liu MY, Chen LY, Guo YL. Lead-induced changes in spermatozoa function and metabolism. J. Toxicol. Environ. Health 1998;55:45–64.
Benoff S, Hauser R, Marmar JL, Hurley IR, Centola GM. Metals and male reproductive function: synthesis and suggestions for future study. Under review.
Roggi C, et al. Trace element reference values in tissues from inhabitants of the European Union. IX. Harmonization of statistical treatment: blood cadmium in Italian subjects. Sci. Total Environ. 1995;166:235–43.
Grasmick C, Huel G, Moreau T, Sarmini H. The combined effect of tobacco and alcohol consumption on the level of lead and cadmium in blood. Sci. Total Environ. 1985;41:207–17.
Murthy RC, Saxena DK, Lal B, Chandra SV. Chronic cadmium-ethanol administration alters metal distribution and some biochemicals in rat brain. Biochem. Int. 1989;19:135–43.
Klaassen CD, Waalkes MP, Cantilena LR Jr. Alteration of tissue disposition of cadmium by chelating agents. Environ. Health Perspect. 1984;54:233–42.
Kelley C, Sargent DE, Uno JK. Cadmium therapeutic agents. Curr. Pharm. Design 1999;5:229–40.
Kemsley J. Improving metal detection in drugs. Chem. Eng. News 2008;86:32–4.
USP Ad Hoc Advisory Panel on Inorganic Impurities and Heavy Metals and USP Staff. General chapter on inorganic impurities: heavy metals. Pharmacopeial Forum 2008;34:1345–7.
Pace V, Iannucci E. The importance of vitamins in relation to the presence of heavy metals in food. Oanminerva Med. 1994;36:80–2.
Gerhard I, Monga B, Waldbrenner A, Runnebaum B. Heavy metals and fertility. J. Toxicol. Environ. Health 1998;54:593–611.
Gunn SA, Gould TC, Anderson WA. Zinc protection against cadmium injury to rat testis. Arch. Pathol. 1961;71:274–81.
Tam JW, Dennehy CE, Ko R, Tsourounis C. Analysis of ephedra-free labeled dietary supplements sold in the San Francisco Bay area in 2003. J. Herb. Pharmacother. 2006;6:1–19.
Jurasovic J, Cvitkovic P, Pizent A, Colak B, Telisman S. Semen quality and reproductive endocrine function with regard to blood cadmium in Croatian male subjects. Biometals 2004;17:735–43.
Chia SE, Xu B, Ong CN, Tsakok FM, Lee ST. Effect of cadmium and cigarette smoking on human semen quality. Int. J. Fertil. Menopausal Stud. 1994;39:292–8.
Matsuoka M, Igisu H. Cadmium induces phosphorylation of p53 at serine in MCF-7 cells. Biochem. Biopys. Res. Commun. 2001;282:1120–5.
Zevin S, Benowitz NL. Drug interactions with tobacco smoking: an update. Clin. Pharmacokinet. 1999;36:425–38.
Larsen L, et al. Computer-assisted semen analysis parameters as predictors for fertility of men from the general population. Hum. Reprod. 2000;15:1562–7.
Aziz N, et al. Novel associations between sperm reactive oxygen species production, sperm morphological defects, and the sperm deformity index. Fertil. Steril. 2004;81:349–54.
Tremellen T. Oxidative stress and male infertility-a clinical perspective. Hum. Reprod. Update 2008;14:243–58.
Agarwal A, Allamaneni SSR, Nallella KP, George AT, Mascha E. Correlation of reactive oxygen species levels with the fertilization rate after in vitro fertilization: a qualified metaanalysis. Fertil. Steril. 2005;84:228–31.
Stone BA, Vargyas JM, Ringler GE, Stein AL, Marrs RP. Determinants of the outcome of intrauterine insemination: analysis of outcomes of 9963 consecutive cycles. Am. J. Obstet. Gynecol. 1999;180:1522–34.
Achard V, et al. Optimization of artificial inseminations with donor semen: a four-year experience. Gynecol. Obstet. Fertil. 2005;33:877–83.
Miller DC, et al. Processed total motile sperm count correlates with pregnancy outcome after intrauterine insemination. Urology 2002;60:497–501.
Ombelet W, et al. Semen quality and intrauterine insemination. Reprod. BioMed. Online 2003;7:485–92.
Beltsos AN, Fisher S, Uhler ML, Clegg ED, Zinaman M. The relationship of the postcoital test and semen characteristics to pregnancy rates in 200 presumed fertile couples. Int. J. Fertil. Menopausal Stud. 1996;41:405–11.
Guzick DS, et al. Sperm morphology, motility, and concentration in fertile and infertile men. N. Engl. J. Med. 2001;345:1388–93.
Balen AH, Rutherford AJ. Management of infertility. BMJ 2007;335:608–11.
Zenzes MT. Cigarette smoking as a cause of delay in conception. Reprod. Med. Rev. 1995;4:189–205.
Vahter M, Berglund M, Akesson A, Liden C. Metals and women’s health. Environ. Res. 2002;88:145–55.
Vahter M, Akesson A, Liden C, Ceccatelli S, Berglund M. Gender differences in the disposition and toxicity of metals. Environ. Res. 2007;104:85–95.
Nishijo M, Satarug S, Honda R, Tsuritani I, Aoshima K. The gender differences in health effects of environmental cadmium exposure and potential mechanisms. Mol. Cell. Biochem. 2004;255:87–92.
Baecklund M, Pedersen NL, Bjorkman L, Vahter M. Variation in blood concentrations of cadmium and lead in the elderly. Environ. Res. 1999;80:222–30.
Bjorkman L, Vahter M, Pedersen NL. Both the environment and genes are important for concentrations of cadmium and lead in blood. Environ. Health Perspect. 2000;108:719–22.
Gunn SA, Gould TC, Anderson WAD. Strain differences in susceptibility of mice and rats to cadmium-induced testicular damage. J. Reprod. Fertil. 1965;10, 273–275.
Taylor BA, Heiniger HJ, Meier H. Genetic analysis of resistance to cadmium-induced testicular damage in mice. Proc. Soc. Exp. Biol. Med. 1973;143:629–33.
King LM, Anderson MB, Sikka SC, George WJ. Murine strain differences and the effects of zinc on cadmium concentration in tissues after acute cadmium exposure. Arch. Toxicol. 1998;72:650–5
King LM, Banks WA, George WJ. Differences in cadmium transport to the testes, epididymis, and brain in cadmium-sensitive and -resistant murine strains 129/J and A/J. J. Pharmacol. Exp. Therapeut. 1999;289:825–30.
King LM, Banks WA, George WJ. Differential zinc transport into testis and brain of cadmium-sensitive and -resistant murine strains. J. Androl. 2000;21:656–63.
Dalton TP, et al. Refining the mouse chromosomal location of Cdm, the major gene associated with susceptibility to cadmium-induced testicular necrosis. Pharmacogenetics 2000;10:141–51.
Benoff S, et al. Voltage-dependent calcium channels in mammalian spermatozoa revisited. Front. Biosci. 2007;12:1420–49.
Benoff S, Marmar JL, Hurley IR. Molecular and other predictors for infertility in patients with varicoceles. Front. Biosci. 2009;14:3641–72.
Goodwin LO, et al. Alternative splicing of exons in the alpha-1 subunit of the rat testis voltage-dependent calcium channel generates germ-line specific dihydropyridine binding sites. Mol. Hum. Reprod. 1998;4:215–26.
Goodwin LO, Karabinus DS, Pergolizzi RG, Benoff S. L-type voltage-dependent calcium channel alpha-1C subunit mRNA is present in ejaculated human spermatozoa. Mol. Hum. Reprod. 2000;6:127–36.
Benoff S, Millan C, Hurley IR, Napolitano B, Marmar JL. Bilateral increased apoptosis and bilateral accumulation of cadmium in infertile men with left varicocele. Hum. Reprod. 2004;19:616–627.
Revis NW, Zinsmeister AR, Bull R. Atherosclerosis and hypertension induction by lead and cadmium ions: an effect prevented by calcium ion. Proc. Nat. Acad. Sci. U. S. A. 1981;78:6494–8.
Schroeder HA, Vinton WH. Hypertension induced in rats by small doses of cadmium. Am. J. Physiol. 1962;202:515–8.
Niwa A, Suzuki A. Effects of cadmium on the tension of isolated rat aorta (a possible mechanism for cadmium-induced hypertension). J. Toxicol. Sci. 1982;7:51–60.
Evans DH, Weingarten K. The effect of cadmium and other metals on vascular smooth muscle of the dogfish shark, Squalus acanthias. Toxicology 1990;61:275–81.
Nolan CV, Shaikh ZA. The vascular endothelium as a target tissue in acute cadmium toxicity. Life Sci. 1986;39:1403–9.
Chiquoine AD. Observation on the early events of cadmium necrosis of the testis. Anat. Rec. 1964;149:23–35.
Dalton TP, et al. Identification of mouse SLC39A8 as the transporter responsible for cadmium-induced toxicity in the testis. Proc. Natl. Acad. Sci. U. S. A. 2005;102:3401–6.
Wang B, et al. Enhanced-cadmium-induced testicular necrosis and renal proximal tubular damage caused by gene-dose increase in a Slc39a8-transgenic mouse line. Am. J. Physiol. Cell Physiol. 2007;292:1523–35.
Martin LJ, et al. FK506, a calcineurin inhibitor, prevents cadmium-induced testicular toxicity in mice. Toxicol. Sci. 2007;100:474–85.
Acknowledgments
The human studies described were supported by grants ES06100 and ES10496 to S Benoff from the National Institute of Environmental Health Sciences, National Institutes of Health, Bethesda, Maryland, and grant OH07330 to S Benoff from the National Institute of Occupational Safety and Health, Centers for Disease Control and Protection, Atlanta, Georgia.
Creation of the cadmium-exposed Wistar rat model was supported by a Faculty Research Award to S Benoff from The Feinstein Institute for Medical Research.
The authors thank DL Rosenfeld, MD; GM Scholl, MD; A Hershlag, MD; and GW Cooper, PhD, for contribution of clinical findings; ML Lesser, PhD, for additional statistical input; and Z Jing Zhang, MD; P Guhring, RN; D Mercerod, RN; A Jacob, PhD; M Barcia, MA; S Canaris, MA; L Yuan, MS; G Kvapil, BS; and D Liotta, MLT, for their technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Benoff, S., Hauser, R., Marmar, J.L. et al. Cadmium Concentrations in Blood and Seminal Plasma: Correlations with Sperm Number and Motility in Three Male Populations (Infertility Patients, Artificial Insemination Donors, and Unselected Volunteers). Mol Med 15, 248–262 (2009). https://doi.org/10.2119/molmed.2008.00104
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2119/molmed.2008.00104