Abstract
Ischemia and reperfusion injury is a dynamic process that involves multiple organ systems in various clinical states including transplantation, trauma, and surgery. Research into this field has identified key molecular and signaling players that mediate, modulate, or augment cellular, tissue, and organ injury during this disease process. Further elucidation of the molecular mechanisms should provide the rationale to identify much-needed novel therapeutic options to prevent or ameliorate organ damage due to ischemia and reperfusion in clinics.
Similar content being viewed by others
Introduction
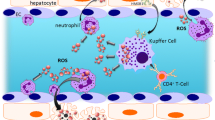
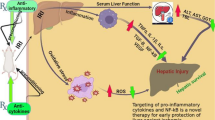
Ischemia and reperfusion injury (IRI) represents a complex series of events that result in cellular and tissue damage. It involves the transient deprivation of blood flow and oxygen, and the return of blood flow during reperfusion with concomitant release of oxygen-free radicals (OFR), cytokines/chemokines, and upregulation of adhesion molecules with consequent cellular and organ dysfunction (Figure 1). Although organ IRI occurs in many clinical settings, including trauma, organ transplantation, myocardial infarction, and stroke, its outcomes remain controversial. Some argue against the significance of lethal reperfusion distinct from ischemic injury, stating that reperfusion merely worsens the initial injury caused by blood deprivation, rather than independently mediating tissue and cellular damage (1). Other data, using therapeutics initiated at time of reperfusion, suggest changes in cell and tissue survival attributed to mitigating lethal reperfusion. Nevertheless, the process of ischemia and reperfusion and the subsequent cellular and organ damage are important to many fields of modern medicine. The following describes the current understanding of molecular mechanisms involved in IRI, primarily in the liver, and their putative therapeutic implications.
Ischemia-reperfusion injury in the liver involves the initial production of oxygen-free radicals and damage-associated molecules with the subsequent involvement of various molecular and cellular cascades, including the Toll-like receptor 4 system, heme oxygenase system, and leukocyte-sinusoidal endothelial cross-talk interactions. Activation of downstream mediators enables the balance between pro-inflammatory and anti-inflammatory responses and the transition from innate to adaptive immune response.
IRI and the Liver
Liver IRI occurs in the absence of exogenous antigens in various clinical settings, such as transplantation, hepatectomy for cancer, and shock. In transplantation, the modified “injury hypothesis” (2,3) states that IRI to the organ activates a cascade of innate-dominated pro-inflammatory immune responses, which then trigger the adaptive immune response that culminates in transplant rejection. The outcome of the transplanted organ is significantly affected by its initial exposure to reactive oxygen species (ROS), damage-associated molecules, and local chemical milieu. Indeed, liver IRI has been reported to cause up to 10% of early organ dysfunction, leading to the higher episodes of both acute and chronic rejection (4). The following review outlines key events in the pathophysiology of liver IRI, including the toll-like receptor (TLR) system, OFRs, the heme oxygenase (HO) system, and leukocyte cascades. Other mechanisms highly involved in liver IRI include tissue anoxia with depletion of adenosine triphosphate and mitochondrial dysfunction (5,6).
TLR system
The sentinel TLRs, mammalian homologs of the Drosophila Toll proteins, play a critical role in innate host defense against microorganisms by recognizing conserved pathogen-associated molecular patterns (7). There are currently 13 known members (11 in humans) of the TLR system, binding a diverse array of bacterial, viral, and fungal molecular patterns (8). TLRs are expressed by a variety of cell types, including macrophages, monocytes, and dendritic cells (DCs). TLR activation leads to the production of pro-inflammatory cytokine programs and upregulation of costimulatory molecules (Figure 2).
In liver ischemia reperfusion injury, the Toll-like receptor 4 (TLR4) system is activated by endogenous and exogenous ligands leading to downstream signal transduction and upregulation of chemokines/cytokines, interferon (IFN)-inducible genes, and costimulatory molecules. These pathways result in full engagement of the innate immune response, transitions to the adaptive immune response, and ultimate hepatocellular damage.
TLR4, one of the best-characterized TLRs, was initially identified as the cellular receptor recognizing the Gramnegative bacterial cell wall product lipopolysaccharide (LPS). Native proteins (endogenous ligands), however, have also been found to engage TLR4 and elicit immune responses in the absence of infection (9). Although endotoxin contamination has never been excluded, putative endogenous ligands/agonists include extracellular matrix proteins (10) (such as fibronectin extra domain A, fibrinogen, hyaluronic acid, heparan sulfate), β defensin, heat-shock proteins (hsp) (11,12), high-mobility group box-1 protein (HMGB-1) (13), S100 proteins (10), and heme (14). During liver IR, portal vein occlusion results in the congestion of the intestinal wall, leading to the release of gut-derived molecules, including endotoxin, into the bloodstream. Although gut-derived LPS certainly contributes to IR, it may not actually trigger the intrahepatic inflammation cascade. Indeed, recent studies have provided definitive proof that endogenous TLR4 ligands generated during liver IR may also trigger local inflammation that culminates in hepatocellular damage (15).
The signaling via TLR4 is critical in liver IRI, as TLR4-deficient (knockout) mice remain fully protected (16). Liver IRI proceeds through the myeloid differentiation primary response gene 88 (MyD88)-independent and the interferon regulatory factor-3 (IRF-3)-dependent pathway downstream of TLR4 (16). Moreover, liver IRI depends on functional TLR4 activation on actively phagocytic nonparenchymal cells, as evidenced by hepatoprotection seen in experiments using chimeric mice generated by adoptive transfer of TLR4-/- bone marrow into wild-type mice (17). TLRs have important roles in the pathophysiology of IRI in other organ systems. In a neonatal murine model of small-bowel IRI, TLR2-deficient mice sustained greater intestinal damage (18); in contrast, TLR2 deficiency was shown to be protective in a murine renal IRI model (19), and involves both MyD88-dependent and -independent signaling pathways (20). However, a recent study points to TLR4-and selective MyD88-mediated signaling to play the dominant role in IRI innate immune response that leads to renal damage (21). Hence, TLR4 expression was upregulated on tubular epithelial cells and infiltrating leukocytes after warm IRI, whereas the absence of TLR4 and MyD88 resulted in renal protection with decreased PMN/macrophage accumulation and decreased expression of pro-inflammatory molecules. In addition, chimeric mice with TLR4 deficiency, specifically in renal parenchymal cells, had significantly less dysfunction and tubular cell damage compared with wild-type renal parenchyma with reconstituted TLR4-/- bone marrow (21). These studies enable comparison of liver IRI with the kidney, such that both depend on TLR4, with liver injury dominated by the MyD88-independent axis and renal IRI mediated by the MyD88-dependent pathway. Additionally, the TLR system has been shown to have a highly important role in myocardial IRI. Indeed, TLR4 deficiency has been shown to decrease infarct size and extent of inflammation (22–24) without changing myocardial function (23). Protection against myocardial IRI in TLR4 knockout mice may proceed through a PI3K/Akt-dependent mechanism (24). Downstream of TLR4, the MyD88-dependent pathway has been shown to be important; blockade of MyD88 signaling resulted in PI3K/Akt pathway activation and nuclear factor-κB (NF-κB) pathway downregulation (25).
Given the ubiquitous functions of TLR signaling and the importance of specific TLR receptor subtypes in animal models of IRI, modulation of the TLR system, through alterations of its ligand-receptor interaction or downstream signaling cascades, may provide novel and exciting options for strategic therapies that mitigate IR-mediated organ damage.
OFRs and Scavengers
Oxygen free radicals are considered one of the most significant components of cell and tissue damage during ischemia and reperfusion. Various cell types generate OFRs, including Kupffer cells (resident liver macrophage) (26) and polymorphoneutrophils (PMNs) (27). Kupffer cells are thought to initiate OFRs and IRI, which is then accentuated by PMNs, resulting in further hepatocyte damage (28). OFRs are produced by xanthine oxidase and damaged mitochondria (28), and include hydrogen peroxide, superoxide anion, and hydroxyl. OFRs have multiple roles in the pathogenesis of liver IRI. These include direct oxidation of cellular components and lipids (lipid peroxidation) (29), activation of inflammatory gene transcription (30), and possible activation of the innate immune response through the TLR system. Indeed, protein oxidation results in the activation of heat shock proteins that subsequently activate the TLR system as mentioned above. OFRs can also generate potentially harmful compounds, such as peroxynitrite, that can potently induce cell death and enhance NF-κB-mediated pro-inflammatory responses (31).
Experimental interventions to reduce OFRs have attenuated liver IR injury in various models. OFR scavengers (32,33) and inhibitors (34), and delivery of antioxidant enzyme genes (35), minimized liver IRI. Adenovirus-mediated gene delivery of the cytosolic Cu/Zn superoxide dismutase gene in a rat marginal liver transplant model reduced OFR production and reduced liver injury (36). The use of manganese-porphyrin (MnP) complexes with superoxide dismutase (SOD) reduced liver IRI in murine models (37), as did administration of α-lipoic acid with OFR-scavenging properties (38). Delivery of newer antioxidant agents has similarly mitigated liver IRI through scavenger effects (39–43). In summary, molecular therapies to reduce IRI will directly or indirectly modulate both the production and metabolism of OFRs.
Nitric Oxide (NO)
NO has a significant role in the microcirculation and organ IRI. NO is produced from l-arginine by nitric oxide synthase (NOS) and exists in both inducible (iNOS) and constitutive forms, such as endothelial NOS (eNOS) and neuronal NOS (44). NO has diverse functions, including inhibition of platelet aggregation, regulation of the microvasculature, and inhibition of caspase activity to prevent apoptosis (45,46). Studies using transgenic overexpression of NO and activators of NO synthase demonstrate cell and tissue protection during IRI. Administration of tetrahydrobiopterin, an important coenzyme of nitric oxide synthase, increased iNOS and eNOS expression, attenuating liver IRI (47,48). NO produced by eNOS is generally considered hepatoprotective (49–51). In a murine liver transplant model, donor eNOS was found to attenuate liver IRI through various mechanisms, including possible vasodilation and reduced macrophage infiltration (52). Additionally, eNOS may exert its hepatoprotective function through the soluble guanylyl cyclase-cGMP pathway independent of HO-1 (53).
The role of iNOS is more controversial. It is reported to have beneficial effects to attenuate organ damage due to IR (49,54), but alternatively, NO expression may exert harmful effects. NO production through iNOS is known to cause oxidative damage by interacting with superoxide anion—leading to the production of peroxynitrite, a potent inducer of cell death (31). Peroxynitrite itself has dual effects, with possible hepatoprotection through decreased leukocyte adhesion and infiltration (55). Results from a porcine liver transplant model suggest that IRI may be triggered by iNOS in Kupffer cell and PMNs (56). Similarly, steatotic rat livers subjected to IR show upregulation of iNOS and endothelin (ET-1), suggesting an important role in regulation of sinusoidal perfusion (57). Inhibition of iNOS may exert beneficial biological effects. Indeed, use of iNOS inhibitors, such as FK330 (FR260330), reduced leukocyte activation, hepatic apoptosis, and overall liver IRI in a rat liver transplant model (58). Another iNOS inhibitor, ONO-1714, similarly attenuated liver IRI (59). Collectively, data suggest a balance between the quantity, duration, and timing of NO expression in liver IRI.
Interventions that increase NO availability have also been shown to be beneficial in various experimental models. Nitrite is an intrinsic signaling molecule that is reduced to NO during ischemia, and has been shown to potently limit liver and cardiac IRI (60). Arginine is a substrate for NO production, and hepatoprotection from the l-arginine-NO synthase pathway has been established in animal IRI models (61,62). Treatment with the arginase inhibitor nor-NOHA resulted in reduced IRI in a liver transplant model, likely through partial restoration of otherwise depleted arginine levels (63).
HO System
HO, a rate-limiting enzyme, catalyzes the degradation of heme into free iron, carbon monoxide (CO), and biliverdin. HO exists in two forms: the oxidative stress-inducible HO-1 (also known as hsp32) and the constitutive isozyme HO-2 (64). HO-1 overexpression, strongly cytoprotective in IRI, mediates anti-inflammatory and anti-apoptotic functions, likely through the effects of various byproducts including biliverdin, bilirubin, ferritin, and CO (Table 1).
HO-1 upregulation using Ad-HO-1 gene transfer, or the HO-1 inducer cobalt protoporphyrin (CoPP), reduced liver damage in steatotic rat liver models of ex vivo cold ischemia followed by reperfusion or isotransplantation (65,66). Moreover, CoPP-induced HO-1 overexpression mitigated warm liver IRI in mice by downregulating STAT-1 activation and CXCL-10 mRNA expression (a key chemokine in liver IRI inflammation cascade), suggesting down-regulation of the type-1 IFN signaling pathway, which is downstream of TLR4 (67). In a rat liver transplant model, adenovirus (Ad)-based HO-1 gene transfer suppressed local expression of iNOS, inhibited caspase-3 expression, and resulted in overall improved graft survival (68). Ad-HO-1 overexpression also prevented CD95/FasL-mediated apoptosis, thereby improving liver function (69,70). Recently, a warm liver IRI model using wild-type and heterozygous HO-1-deficient (HO-1+/−) mice demonstrated that basal HO-1 levels are more important than the upregulation of HO-1 in response to IR (71). Pretreatment with inhaled anesthetic isoflurane has also shown hepatoprotection in liver IRI, through increasing local HO-1 mRNA/protein expression and HO-1 enzyme activity (72).
Products of HO degradation have also been shown to attenuate IRI. Adjunctive biliverdin therapy reduced hepatocellular damage in well-established ex vivo and in vivo models of hepatic IRI, likely through decreased endothelial expression of cellular adhesion molecules, inhibition of proinflammatory cytokines, and upregulation of anti-apoptotic pathways (73–75). Exogenous biliverdin therapy decreased liver damage in a small-for-size rat transplant model, possibly through inhibition of pro-apoptotic and pro-inflammatory JNK/AP-1 signaling (76).
CO is another heme degradation product with important anti-inflammatory and cytoprotective effects. Exogenous therapy with CO has been shown to reduce IRI in many organ systems including the heart, lung, intestine, and kidney (77). Inhaled CO treatment in an ex vivo rat liver IRI model (78), and liver transplant model, suppressed early proinflammatory gene expression and neutrophil infiltration, with resultant amelioration of organ damage (77).
Owing to its potent cytoprotective properties, the HO system may be a useful strategy to minimize IRI in transplantation, shock, and other low-flow states. HO-1 induction through pharmacological means (such as CoPP), gene therapy, or delivery of downstream products such as CO or biliverdin may reduce cell and tissue damage. Use of the HO system in the clinical setting is limited by many factors, such as choice of therapeutic window and the ability to control constitutively active gene delivery products.
Leukocytes and Liver IRI
The liver is comprised of parenchymal cells (hepatocytes) and nonparenchymal cells, such as endothelial cells, stellate cells, Kupffer cells, macrophages, and lymphocytes—such as CD4+ and CD8+ T cells (conventional T cells), unconventional T cells (NKT, γδ T cells), natural killer (NK) cells, and B cells (79). The liver also has resident antigen-presenting cells such as Kupffer cells and DCs. Liver leukocytes are located throughout, both within the parenchyma and within the portal tracts (79). Leukocytes have an important role at the interface of portal blood flow with exposure to pathogenic and nonpathogenic antigens. Moreover, they are highly involved in liver IRI and function both to amplify the molecular pathways, and to directly cause cellular damage (80,81).
A number of mechanisms may account for leukocyte infiltration during liver IRI. One major mechanism is through the adhesion cascade that consists of physical leukocyte-endothelial cross-talk interactions during reperfusion. It is known that liver IRI involves microcirculatory flow disturbances with involvement of endothelial cell adhesion and leukocyte tethering and infiltration. Local leukocyte infiltration depends on both extravasation across the vascular endothelium and migration through the extracellular matrix. Leukocyte recruitment involves the initial attachment, or tethering, and rolling of leukocytes on the sinusoidal endothelium mediated through the selectin family (P-selectin, E-selectin, and L-selectin). The principle selectin ligand is P-selectin glycoprotein ligand (PSGL-1), a 220-kDa mucin-like ligand that binds all three selectins and is located on the surface of most leukocyte subclasses (82). The blockade of P-selectin and PSGL-1 protects against IRI in liver (83–87), kidney (88,89), and small intestine (90–92) animal models, and is currently being tested in renal and liver transplant patients.
In addition to P-selectin, phosphatidylserine (PS) appears to have an important role in leukocyte diapedesis and migration. Anoxia and reoxygenation cause externalization of PS in sinusoidal endothelial cells (ECs) (93), which promotes attachment of leukocytes and platelets to the microvasculature and impairs blood flow. Molecules such as diannexin, a 73-kDa homodimer of human annexin V (94), bind PS and minimize EC activation (95). By minimizing leukocyte and platelet targeting of the liver, diannexin indirectly suppresses inflammatory responses and decreases apoptosis, thereby mitigating liver IRI (96) and improving allograft survival in a rat liver transplant model (95). Additionally, matrix proteases facilitate the movement of leukocytes along the vasculature. Matrix metalloproteinase (MMP)-9, a gelatinase family member, is highly expressed after IRI (97). Its blockade results in impaired myeloperoxidase activation and decreased leukocyte accumulation (98). MMP-9 deficiency reduces liver IR injury (98) and improves survival with attenuation of TNF-α release and endothelial CD62P expression (99).
Leukocytes are also targeted to the liver during IRI. Systemic CD4+ T cells are recruited to the liver within the first hour of reperfusion (100). The exact mechanism of this process is under investigation and may proceed via ligation of the TLR4 system, upregulation of CXCL10, and chemotaxis of CXCR3+ CD4+ T cells (101). Various chemokines/cytokines are expressed during the acute phase of IRI and include CXCL10, RANTES, MCP-1, MIP-1α, MIP-1β, and MIP-2, among others (102,103). These molecules are chemotactic to PMNs, which accumulate in the liver and have a significant role in the subacute injury phase. Additionally, CD4+ T cells release IL-17, which likely further mediates neutrophil chemotaxis (80). Indeed, the absence of CD4+ T cells results in reduced recruitment of PMNs and protection from IRI (100,104,105).
Clinical Applications
Basic research using animal models has elucidated dominant molecular pathways important in the pathogenesis of liver IRI. Application of this knowledge is underway through clinical trials that investigate therapeutic modalities to reduce IRI (Table 2). As described above, animal models that increase NO availability have reduced liver IRI. A small (n = 10) prospective randomized controlled trial was conducted to evaluate the use of inhaled NO on liver function and patient outcome after liver transplantation (106). In this study, liver transplant patients were treated with placebo (nitrogen) or inhaled NO (80 parts per million) during the perioperative period. The NO treatment group had earlier improved graft function, reduced hepatocyte apoptosis, and shorter length of hospital stay. Limitations to the study included small sample size and use of a single liver biopsy at one hour after reperfusion to evaluate inflammatory changes and hepatocyte apoptosis.
Apoptosis and hepatocellular damage are significant end points in the pathogenesis of liver IRI. Indeed, multiple signaling pathways in IRI culminate with apoptosis and organ dysfunction. Recently, IDN-6556, a pan-caspase inhibitor (PCI), was applied in liver transplant patients to evaluate its efficacy to mitigate liver injury during cold and warm IR (107). PCI was administered locally to the donor organ during cold storage and systemically to the transplant recipient for 24–48 h posttransplant. Organ storage with PCI resulted in both reduced early liver IR-mediated apoptosis and decreased injury. Rates of acute cellular rejection were similar between groups. Systemic PCI delivery, however, reduced the beneficial effects of PCI administered during cold storage alone. This was attributed to neutrophil accumulation and local inflammation due to decreased neutrophil apoptosis with systemic intravenous PCI delivery (107).
In addition to therapies aimed at preventing hepatocyte apoptosis, anti-inflammatory agents have been investigated for protection against liver IRI. A recent prospective clinical trial assessed the use of preoperative steroid administration on outcome after hepatectomy (108). Patients (n = 36) treated with preoperative methylprednisolone (500 mg) before induction of anesthesia had lower serum transaminases, coagulation parameters, and inflammatory cytokines (TNF-α and IL-6). Of interest, the protective role of preoperative steroid treatment was more significant in patients with larger liver resection and greater ischemic time. A second study (n = 25) that used methylprednisolone (30 mg/kg) 30 min before liver resection resulted in reduced IL-6 levels without significant improvement in short-term outcomes (109).
The efficacy of other immunosuppressive agents to reduce liver IRI has been examined. Tacrolimus administered to livers in a flush-solution before transplantation resulted in improved early organ function and decreased hepatocellular damage (110). Various molecular pathways may have contributed to the beneficial effects, including anti-inflammation and preservation of mitochondrial function (110). Other studies have investigated the use of antioxidants, such as N-acetyl cysteine (NAC) used in a small randomized study (n = 9) during donor hepatectomy (111). There was no protective effect on acute cellular rejection or the extent of liver IRI, though the study was limited by small sample size.
Thymoglobulin (TG), a polyclonal antibody induction agent used in kidney, pancreas, and liver transplantation, was recently shown to reduce renal IRI and to improve allograft function after cadaveric renal transplantation (112). TG reduced renal delayed graft function and decreased length of hospital stay in renal transplantation when given intraoperatively before reperfusion (112). Given these beneficial effects in the kidney, a prospective trial was conducted to evaluate the efficacy of TG to reduce liver IRI during transplantation. TG was shown to reduce liver IRI and to improve allograft function (113). The mechanism of action for this therapeutic effect is not completely known, but may involve blockade of adhesion molecules that activate downstream signaling integral to IRI or depletion of T lymphocytes.
In summary, recent clinical trials have tested the ability of immunomodulatory agents to improve patient outcomes after IRI. Knowledge of the molecular and signaling pathways established through basic research has enabled initial application of these novel therapeutic options to minimize IRI and improve medical treatments. Considerable work remains to address this complex problem in clinical practice.
Conclusion
Liver IRI represents a continuum of complex processes that involve multiple cellular and molecular pathways. This review focused on liver IRI, primarily in the context of transplantation. Given the scope of IRI and its wide applicability to many fields in medicine, continued efforts are necessary to understand the pathophysiology of this disease process and minimize its detrimental effects. Indeed, translation of this knowledge to the clinical setting will allow much-needed improvements in patient survival and outcomes in many disease states, including trauma and transplantation.
References
Yellon DM, Hausenloy DJ. (2007) Myocardial reperfusion injury. N. Engl. J. Med. 357:1121–35.
Land W, et al. (1994) The beneficial effect of human recombinant superoxide dismutase on acute and chronic rejection events in recipients of cadaveric renal transplants. Transplantation 57:211–7.
Land WG. (2005) The role of postischemic reperfusion injury and other nonantigen-dependent inflammatory pathways in transplantation. Transplantation 79:505–14.
Fondevila C, Busuttil RW, Kupiec-Weglinski JW. (2003) Hepatic ischemia/reperfusion injury: a fresh look. Exp. Mol. Pathol. 74:86–93.
Lemasters JJ, Nieminen AL, Qian T, Trost LC, Herman B. (1997) The mitochondrial permeability transition in toxic, hypoxic and reperfusion injury. Mol. Cell. Biochem. 174:159–65.
Belzer FO, Southard JH. (1988) Principles of solid-organ preservation by cold storage. Transplantation 45:673–6.
Takeda K, Kaisho T, Akira S. (2003) Toll-like receptors. Ann. Rev. Immunol. 21:335–76.
Hopkins PA, Sriskandan S. (2005) Mammalian Toll-like receptors: to immunity and beyond. Clin. Exp. Immunol. 140:395–407.
Tsan MF, Gao B. (2004) Endogenous ligands of Toll-like receptors. J. Leukocyte Biol. 76:514–9.
Foell D, Wittkowski H, Roth J. (2007) Mechanisms of disease: a ‘DAMP’ view of inflammatory arthritis. Nat. Clin. Pract. Rheumatol. 3:382–90.
Roelofs MF, et al. (2006) Identification of small heat shock protein B8 (HSP22) as a novel TLR4 ligand and potential involvement in the pathogenesis of rheumatoid arthritis. J. Immunol. 176:7021–7.
Ohashi K, Burkart V, Flohe S, Kolb H. (2000) Cutting edge: heat shock protein 60 is a putative endogenous ligand of the toll-like receptor-4 complex. J. Immunol. 164:558–61.
Levy RM, et al. (2007) Systemic inflammation and remote organ injury following trauma require HMGB1. Am. J. Physiol. Regul. Integr. Comp. Physiol. 293:R1538–44.
Figueiredo RT, et al. (2007) Characterization of heme as activator of Toll-like receptor 4. J. Biol. Chem. 282:20221–9.
Zhai Y, Qiao B SH, Gao F, Busuttil RW, Cheng G, Platt JL, Volk HD, Kupiec-Weglinski JW. Evidence for the pivotal role of endogenous TLR4 ligands in liver ischemia and reperfusion injury. Transplantation (In Press).
Zhai Y, et al. (2004) Cutting edge: TLR4 activation mediates liver ischemia/reperfusion inflammatory response via IFN regulatory factor 3-dependent MyD88-independent pathway. J. Immunol. 173:7115–9.
Tsung A, et al. (2005) Hepatic ischemia/reperfusion injury involves functional TLR4 signaling in non-parenchymal cells. J. Immunol. 175:7661–8.
Aprahamian CJ, Lorenz RG, Harmon CM, Dimmit RA. (2008) Toll-like receptor 2 is protective of ischemia-reperfusion-mediated small-bowel injury in a murine model. Pediatr. Crit. Care Med. 9:105–9.
Leemans JC, et al. (2005) Renal-associated TLR2 mediates ischemia/reperfusion injury in the kidney. J. Clin. Invest. 115:2894–903.
Shigeoka AA, et al. (2007) TLR2 is constitutively expressed within the kidney and participates in ischemic renal injury through both MyD88-dependent and -independent pathways. J. Immunol. 178:6252–8.
Wu H, et al. (2007) TLR4 activation mediates kidney ischemia/reperfusion injury. J. Clin. Invest. 117:2847–59.
Oyama J, et al. (2004) Reduced myocardial ischemia-reperfusion injury in toll-like receptor 4-deficient mice. Circulation 109:784–9.
Kim SC, et al. (2007) Toll-like receptor 4 deficiency: smaller infarcts, but no gain in function. BMC Physiol. 7:5.
Hua F, et al. (2007) Protection against myocardial ischemia/reperfusion injury in TLR4-deficient mice is mediated through a phosphoinositide 3-kinase-dependent mechanism. J. Immunol. 178:7317–24.
Hua F, et al. (2005) Blocking the MyD88-dependent pathway protects the myocardium from ischemia/reperfusion injury in rat hearts. Biochem. Biophys. Res. Commun. 338:1118–25.
Jaeschke H, Farhood A. (1991) Neutrophil and Kupffer cell-induced oxidant stress and ischemia-reperfusion injury in rat liver. Am. J. Physiol. 260:G355–62.
Jaeschke H, Smith CW, Clemens MG, Ganey PE, Roth RA. (1996) Mechanisms of inflammatory liver injury: adhesion molecules and cytotoxicity of neutrophils. Toxicol. Appl. Pharmacol. 139:213–26.
Jaeschke H. (2002) Reperfusion injury after warm ischemia or cold storage of the liver: role of apoptotic cell death. Transplant. Proc. 34:2656–8.
Poli G, Cutrin JC, Biasi F. (1998) Lipid peroxidation in the reperfusion injury of the liver. Free Radic. Res. 28:547–51.
Jaeschke H. (2000) Reactive oxygen and mechanisms of inflammatory liver injury. J. Gastroenterol. Hepatol. 15:718–24.
Szabo C, Ischiropoulos H, Radi R. (2007) Peroxynitrite: biochemistry, pathophysiology and development of therapeutics. Nat. Rev. Drug Discov. 6:662–80.
Yuzawa H, et al. (2005) Inhibitory effects of safe and novel SOD derivatives, galactosylated-SOD, on hepatic warm ischemia/reperfusion injury in pigs. Hepatogastroenterology 52:839–43.
Mizoe A, et al. (1997) Preventive effects of superoxide dismutase derivatives modified with monosaccharides on reperfusion injury in rat liver transplantation. J. Surg. Res. 73:160–5.
Ejiri S, Eguchi Y, Kishida A, Kurumi Y, Tani T, Kodama M. (2000) Protective effect of OPC-6535, a superoxide anion production inhibitor, on liver grafts subjected to warm ischemia during porcine liver transplantation. Transplant Proc. 32:318–21.
He SQ, et al. (2006) Delivery of antioxidative enzyme genes protects against ischemia/reperfusion-induced liver injury in mice. Liver Transpl. 12:1869–79.
Lehmann TG, et al. (2000) Gene delivery of Cu/Zn-superoxide dismutase improves graft function after transplantation of fatty livers in the rat. Hepatology (Baltimore) 32:1255–64.
Wu TJ, Khoo NH, Zhou F, Day BJ, Parks DA. (2007) Decreased hepatic ischemia-reperfusion injury by manganese-porphyrin complexes. Free Radic. Res. 41:127–34.
Dulundu E, et al. (2007) Alpha-lipoic acid protects against hepatic ischemia-reperfusion injury in rats. Pharmacology 79:163–70.
Abe T, et al. (2004) A new free radical scavenger, edaravone, ameliorates oxidative liver damage due to ischemia-reperfusion in vitro and in vivo. J. Gastrointest. Surg. 8:604–15.
Ninomiya M, Shimada M, Harada N, Soejima Y, Suehiro T, Maehara Y. (2004) The hydroxyl radical scavenger MCI-186 protects the liver from experimental cold ischaemia-reperfusion injury. Br. J. Surg. 91:184–90.
Ninomiya M, et al. (2002) Beneficial effect of MCI-186 on hepatic warm ischemia-reperfusion in the rat. Transplantation 74:1470–2.
Sepodes B, et al. (2004) Tempol, an intracellular free radical scavenger, reduces liver injury in hepatic ischemia-reperfusion in the rat. Transplant. Proc. 36:849–53.
Yokota R, et al. (2000) A novel hydroxyl radical scavenger, nicaraven, protects the liver from warm ischemia and reperfusion injury. Surgery 127:661–9.
Moncada S, Higgs A. (1993) The L-arginine-nitric oxide pathway. N. Engl. J. Med. 329:2002–12.
Mocellin S, Bronte V, Nitti D. (2007) Nitric oxide, a double edged sword in cancer biology: searching for therapeutic opportunities. Med. Res. Rev. 27:317–52.
Bogdan C. (2001) Nitric oxide and the immune response. Nat. Immunol. 2:907–16.
Hara Y, Teramoto K, Ishidate K, Arii S. (2006) Cytoprotective function of tetrahydrobiopterin in rat liver ischemia/reperfusion injury. Surgery 139:377–84.
Hara Y, et al. (2005) Beneficial effect of tetrahydrobiopterin on the survival of rats exposed to hepatic ischemia-reperfusion injury. Transplant. Proc. 37:442–4.
Kaizu T, et al. (2006) Donor graft adenoviral iNOS gene transfer ameliorates rat liver transplant preservation injury and improves survival. Hepatology (Baltimore) 43:464–73.
Hines IN, Harada H, Flores S, Gao B, McCord JM, Grisham MB. (2005) Endothelial nitric oxide synthase protects the post-ischemic liver: potential interactions with superoxide. Biomed. Pharmacother. 59:183–9.
Varadarajan R, et al. (2004) Nitric oxide in early ischaemia reperfusion injury during human orthotopic liver transplantation. Transplantation 78:250–6.
Theruvath TP, Zhong Z, Currin RT, Ramshesh VK, Lemasters JJ. (2006) Endothelial nitric oxide synthase protects transplanted mouse livers against storage/reperfusion injury: role of vasodilatory and innate immunity pathways. Transplant. Proc. 38:3351–7.
Duranski MR, Elrod JW, Calvert JW, Bryan NS, Feelisch M, Lefer DJ. (2006) Genetic overexpression of eNOS attenuates hepatic ischemia-reperfusion injury. Am. J. Physiol. Heart Circ. Physiol. 291:H2980–6.
Schwentker A, Billiar TR. (2002) Inducible nitric oxide synthase: from cloning to therapeutic applications. World J. Surg. 26:772–8.
Liu P, Xu B, Quilley J, Wong PY. (2000) Peroxynitrite attenuates hepatic ischemia-reperfusion injury. Am. J. Physiol. Cell Physiol. 279:C1970–7.
Kimura H, et al. (2003) Role of inducible nitric oxide synthase in pig liver transplantation. J. Surg. Res. 111:28–37.
Koeppel TA, et al. (2007) Enhanced iNOS gene expression in the steatotic rat liver after normothermic ischemia. Eur. Surg. Res. 39:303–11.
Tsuchihashi S, et al. (2006) FK330, a novel inducible nitric oxide synthase inhibitor, prevents ischemia and reperfusion injury in rat liver transplantation. Am. J. Transplant. 6:2013–22.
Meguro M, et al. (2002) A novel inhibitor of inducible nitric oxide synthase (ONO-1714) prevents critical warm ischemia-reperfusion injury in the pig liver. Transplantation 73:1439–46.
Shiva S, et al. (2007) Nitrite augments tolerance to ischemia/reperfusion injury via the modulation of mitochondrial electron transfer. J. Exp. Med. 204:2089–102.
Yagnik GP, Takahashi Y, Tsoulfas G, Reid K, Murase N, Geller DA. (2002) Blockade of the l-arginine/NO synthase pathway worsens hepatic apoptosis and liver transplant preservation injury. Hepatology (Baltimore) 36:573–81.
Geller DA, Chia SH, Takahashi Y, Yagnik GP, Tsoulfas G, Murase N. (2001) Protective role of the l-arginine-nitric oxide synthase pathway on preservation injury after rat liver transplantation. JPEN J. Parenter. Enteral Nutr. 25:142–7.
Reid KM, et al. (2007) Liver I/R injury is improved by the arginase inhibitor, N(omega)-hydroxy-nor-L-arginine (nor-NOHA). Am. J. Physiol. 292:G512–7.
Maines MD. (1997) The heme oxygenase system: a regulator of second messenger gases. Annu. Rev. Pharmacol. Toxicol. 37:517–54.
Amersi F, et al. (1999) Upregulation of heme oxygenase-1 protects genetically fat Zucker rat livers from ischemia/reperfusion injury. J. Clin. Invest. 104:1631–9.
Kato H, et al. (2001) Heme oxygenase-1 overexpression protects rat livers from ischemia/reperfusion injury with extended cold preservation. Am. J. Transplant. 1:121–8.
Tsuchihashi S, Zhai Y, Bo Q, Busuttil RW, Kupiec-Weglinski JW. (2007) Heme oxygenase-1 mediated cytoprotection against liver ischemia and reperfusion injury: inhibition of type-1 interferon signaling. Transplantation 83:1628–34.
Coito AJ, et al. (2002) Heme oxygenase-1 gene transfer inhibits inducible nitric oxide synthase expression and protects genetically fat Zucker rat livers from ischemia-reperfusion injury. Transplantation 74:96–102.
Ke B, et al. (2002) Heme oxygenase-1 gene transfer prevents CD95/FasL-mediated apoptosis and improves liver allograft survival via carbon monoxide signaling pathway. Transplant. Proc. 34:1465–6.
Ke B, et al. (2002) Heme oxygenase 1 gene transfer prevents CD95/Fas ligand-mediated apoptosis and improves liver allograft survival via carbon monoxide signaling pathway. Hum. Gene Ther. 13:1189–99.
Tsuchihashi S, Livhits M, Zhai Y, Busuttil RW, Araujo JA, Kupiec-Weglinski JW. (2006) Basal rather than induced heme oxygenase-1 levels are crucial in the antioxidant cytoprotection. J. Immunol. 177:4749–57.
Schmidt R, et al. (2007) Heme oxygenase-1 induction by the clinically used anesthetic isoflurane protects rat livers from ischemia/reperfusion injury. Ann. Surg. 245:931–42.
Fondevila C, et al. (2004) Biliverdin therapy protects rat livers from ischemia and reperfusion injury. Hepatology (Baltimore) 40:1333–41.
Fondevila C, et al. (2003) Biliverdin protects rat livers from ischemia/reperfusion injury. Transplant. Proc. 35:1798–9.
Kato Y, et al. (2003) Bilirubin rinse: a simple protectant against the rat liver graft injury mimicking heme oxygenase-1 preconditioning. Hepatology (Baltimore) 38:364–73.
Tang LM, et al. (2007) Exogenous biliverdin ameliorates ischemia-reperfusion injury in small-for-size rat liver grafts. Transplant. Proc. 39:1338–44.
Kaizu T, et al. (2005) Carbon monoxide inhalation ameliorates cold ischemia/reperfusion injury after rat liver transplantation. Surgery 138:229–35.
Amersi F, et al. (2002) Ex vivo exposure to carbon monoxide prevents hepatic ischemia/reperfusion injury through p38 MAP kinase pathway. Hepatology (Baltimore) 35:815–23.
Racanelli V, Rehermann B. (2006) The liver as an immunological organ. Hepatology (Baltimore) 43:S54–62.
Caldwell CC, Tschoep J, Lentsch AB. (2007) Lymphocyte function during hepatic ischemia/reperfusion injury. J. Leukocyte Biol. 82:457–64.
Huang Y, Rabb H, Womer KL. (2007) Ischemia-reperfusion and immediate T cell responses. Cell. Immunol. 248:4–11.
Vachino G, et al. (1995) P-selectin glycoprotein ligand-1 is the major counter-receptor for P-selectin on stimulated T cells and is widely distributed in non-functional form on many lymphocytic cells. J. Biol. Chem. 270:21966–74.
Yadav SS, Howell DN, Steeber DA, Harland RC, Tedder TF, Clavien PA. (1999) P-Selectin mediates reperfusion injury through neutrophil and platelet sequestration in the warm ischemic mouse liver. Hepatology (Baltimore) 29:1494–502.
Tsuchihashi S, et al. (2006) Molecular characterization of rat leukocyte P-selectin glycoprotein ligand-1 and effect of its blockade: protection from ischemia-reperfusion injury in liver transplantation. J. Immunol. 176:616–24.
Amersi F, et al. (2002) P-selectin glycoprotein ligand-1 (rPSGL-Ig)-mediated blockade of CD62 selectin molecules protects rat steatotic liver grafts from ischemia/reperfusion injury. Am. J. Transplant. 2:600–8.
Amersi F, et al. (2001) A novel iron chelator in combination with a P-selectin antagonist prevents ischemia/reperfusion injury in a rat liver model. Transplantation 71:112–8.
Dulkanchainun TS, et al. (1998) Reduction of hepatic ischemia/reperfusion injury by a soluble P-selectin glycoprotein ligand-1. Ann. Surg. 227:832–40.
Fuller TF, Sattler B, Binder L, Vetterlein F, Ringe B, Lorf T. (2001) Reduction of severe ischemia/reperfusion injury in rat kidney grafts by a soluble P-selectin glycoprotein ligand. Transplantation 72:216–22.
Singbartl K, Green SA, Ley K. (2000) Blocking P-selectin protects from ischemia/reperfusion-induced acute renal failure. FASEB J. 14:48–54.
Farmer DG, et al. (2002) Improved survival through the reduction of ischemia-reperfusion injury after rat intestinal transplantation using selective P-selectin blockade with P-selectin glycoprotein ligand-Ig. Transplant. Proc. 34:985.
Farmer DG, et al. (2005) Disruption of P-selectin signaling modulates cell trafficking and results in improved outcomes after mouse warm intestinal ischemia and reperfusion injury. Transplantation 80:828–35.
Carmody IC, et al. (2004) P-selectin knockout mice have improved outcomes with both warm ischemia and small bowel transplantation. Transplant. Proc. 36:263–4.
Ran S, Downes A, Thorpe PE. (2002) Increased exposure of anionic phospholipids on the surface of tumor blood vessels. Cancer Res. 62:6132–40.
Kuypers FA, Larkin SK, Emeis JJ, Allison AC. (2007) Interaction of an annexin V homodimer (Diannexin) with phosphatidylserine on cell surfaces and consequent antithrombotic activity. Thromb. Haemost. 97:478–86.
Shen XD, et al. (2007) Diannexin, a novel annexin V homodimer, protects rat liver transplants against cold ischemia-reperfusion injury. Am. J. Transplant. 7:2463–71.
Teoh NC, et al. (2007) Diannexin, a novel annexin V homodimer, provides prolonged protection against hepatic ischemia-reperfusion injury in mice. Gastroenterology 133:632–46.
Moore C, Shen XD, Gao F, Busuttil RW, Coito AJ. (2007) Fibronectin-alpha4beta1 integrin interactions regulate metalloproteinase-9 expression in steatotic liver ischemia and reperfusion injury. Am. J. Pathol. 170:567–77.
Hamada T, Fondevila C, Busuttil RW, Coito AJ. (2008) Metalloproteinase-9 deficiency protects against hepatic ischemia/reperfusion injury. Hepatology (Baltimore) 47:186–98.
Khandoga A, et al. (2006) Matrix metalloproteinase-9 promotes neutrophil and T cell recruitment and migration in the postischemic liver. J. Leukocyte Biol. 79:1295–305.
Zwacka RM, Zhang Y, Halldorson J, Schlossberg H, Dudus L, Engelhardt JF. (1997) CD4+ T-lymphocytes mediate ischemia/reperfusion-induced inflammatory responses in mouse liver. J. Clin. Invest. 100:279–89.
Zhai Y, et al. (2006) CXCR3+CD4+T cells mediate innate immune function in the pathophysiology of liver ischemia/reperfusion injury. J. Immunol. 176:6313–22.
Day YJ, Marshall MA, Huang L, McDuffie MJ, Okusa MD, Linden J. (2004) Protection from ischemic liver injury by activation of A2A adenosine receptors during reperfusion: inhibition of chemokine induction. Am. J. Physiol. 286:G285–93.
Colletti LM, Green ME, Burdick MD, Strieter RM. (2000) The ratio of ELR+ to ELR− CXC chemokines affects the lung and liver injury following hepatic ischemia/ reperfusion in the rat. Hepatology (Baltimore) 31:435–45.
Caldwell CC, Okaya T, Martignoni A, Husted T, Schuster R, Lentsch AB. (2005) Divergent functions of CD4+ T lymphocytes in acute liver inflammation and injury after ischemia-reperfusion. Am. J. Physiol. 289:G969–76.
Rabb H, et al. (2000) Pathophysiological role of T lymphocytes in renal ischemia-reperfusion injury in mice. Am. J. Physiol. Renal Physiol. 279:F525–31.
Lang JD Jr, et al. (2007) Inhaled NO accelerates restoration of liver function in adults following orthotopic liver transplantation. J. Clin. Invest. 117:2583–91.
Baskin-Bey ES, et al. (2007) Clinical trial of the pan-caspase inhibitor, IDN-6556, in human liver preservation injury. Am. J. Transplant. 7:218–25.
Aldrighetti L, et al. (2006) Impact of preoperative steroids administration on ischemia-reperfusion injury and systemic responses in liver surgery: a prospective randomized study. Liver Transpl. 12:941–9.
Muratore A, Ribero D, Ferrero A, Bergero R, Capussotti L. (2003) Prospective randomized study of steroids in the prevention of ischaemic injury during hepatic resection with pedicle clamping. Br. J. Surg. 90:17–22.
St Peter SD, Post DJ, Rodriguez-Davalos MI, Douglas DD, Moss AA, Mulligan DC. (2003) Tacrolimus as a liver flush solution to ameliorate the effects of ischemia/reperfusion injury following liver transplantation. Liver Transpl. 9:144–9.
Khan AW, Fuller BJ, Shah SR, Davidson BR, Rolles K. (2005) A prospective randomized trial of N-acetyl cysteine administration during cold preservation of the donor liver for transplantation. Ann. Hepatol. 4:121–6.
Goggins WC, et al. (2003) A prospective, randomized, clinical trial of intraoperative versus postoperative Thymoglobulin in adult cadaveric renal transplant recipients. Transplantation 76:798–802.
Bogetti D, et al. (2005) Thymoglobulin induction protects liver allografts from ischemia/reperfusion injury. Clin. Transplant. 19:507–11.
Ito K, Ozasa H, Sanada K, Horikawa S. (2000) Doxorubicin preconditioning: a protection against rat hepatic ischemia-reperfusion injury. Hepatology (Baltimore) 31:416–9.
Shen XD, et al. (2002) CD154-CD40 T-cell co-stimulation pathway is required in the mechanism of hepatic ischemia/reperfusion injury, and its blockade facilitates and depends on heme oxygenase-1 mediated cytoprotection. Transplantation 74:315–9.
Redaelli CA, Tian YH, Schaffner T, Ledermann M, Baer HU, Dufour JF. (2002) Extended preservation of rat liver graft by induction of heme oxygenase-1. Hepatology (Baltimore) 35:1082–92.
Berberat PO, et al. (2003) Heavy chain ferritin acts as an antiapoptotic gene that protects livers from ischemia reperfusion injury. FASEB J. 17:1724–6.
Ke B, Shen XD, Lassman CR, Gao F, Busuttil RW, Kupiec-Weglinski JW. (2003) Cytoprotective and antiapoptotic effects of IL-13 in hepatic cold ischemia/reperfusion injury are heme oxygenase-1 dependent. Am. J. Transplant. 3:1076–82.
Shen XD, et al. (2003) Stat4 and Stat6 signaling in hepatic ischemia/reperfusion injury in mice: HO-1 dependence of Stat4 disruption-mediated cytoprotection. Hepatology (Baltimore) 37:296–303.
Ke B, et al. (2004) Gene therapy for liver transplantation using adenoviral vectors: CD40-CD154 blockade by gene transfer of CD40Ig protects rat livers from cold ischemia and reperfusion injury. Mol. Ther. 9:38–45.
Shen XD, et al. (2005) Toll-like receptor and heme oxygenase-1 signaling in hepatic ischemia/reperfusion injury. Am. J. Transplant. 5:1793–800.
Kim YI, Hwang YJ, Song KE, Yun YK, Lee JW, Chun BY. (2002) Hepatocyte protection by a protease inhibitor against ischemia/reperfusion injury of human liver. J. Am. Coll. Surg. 195:41–50.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Vardanian, A.J., Busuttil, R.W. & Kupiec-Weglinski, J.W. Molecular Mediators of Liver Ischemia and Reperfusion Injury: A Brief Review. Mol Med 14, 337–345 (2008). https://doi.org/10.2119/2007-00134.Vardanian
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2119/2007-00134.Vardanian