Abstract
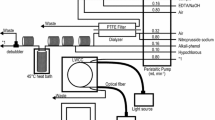
A spectrophotometric method for the determination of total carbonate in water samples was developed. The method is based on the color change of an acid-base indicator in relation to the concentration of permeable gas substances through a membrane. By using a new portable FIA system equipped with a gas-diffusion unit, a highly sensitive and on-site determination of total carbonate in aqueous solutions was investigated. A new color-change system with 4-(2′,4′-dinitrophenylazo)-1-naphthol-5-sulfonic acid (DNN5S) was developed. Absorbance changes of the reagent solution were measured at 450 nm with a light-emitting diode (LED) as a light source. A new type of gas-diffusion unit was used, and was constructed with double tubing: the inner tubing was a micro porous PTFE (polytetrafluoroethylene) tubing (1.0 mm inner diameter and 1.8 mm outer diameter, pore size 2 µm, porosity 50%); the outer tubing was made of glass with 2.0 mm inner diameter. The optimized system conditions were as follows: the sample size was 200 µl, the temperature of the air bath for the gas-diffusion unit was 25°C, and the length of the gas-diffusion unit was 15 cm; each flow rate was 0.3 ml min−1. For measuring carbonate at low concentrations, a method for preparing water with less carbonate was proposed: the carbonate content of the water was decreased down to 5 × 10−7 M. The calibration graph was rectilinear from 1 × 10−6 M to 10−3 M, and the detection limit (corresponding to a signal-to-noise ratio of 3) was 1 × 102212;6 M of carbonate. The relative standard deviation (RSD) of ten measurements of 2.3 × 10−5 M Na2CO3 solution was 1.9%. The total carbonate in various kinds of water (such as river, sea, rain, distilled and ultra purified) was determined.
Similar content being viewed by others
References
S. Motomizu, K. Toei, T. Kuwaki, and M. Oshima, Anal. Chem., 1987, 592, 930.
T. Kuwaki, K. Toei, M. Akiba, M. Oshima, and S. Motomizu, Bunseki Kagaku, 1987, 36, T132.
M. Sanada, M. Oshima, and S. Motomizu, Bunseki Kagaku, 1993, 42, T123.
V. Kuban and P. K. Dasgupta, Talanta, 1993, 40, 831.
P. Linares, M. D. Lugue de Castro, and M. Valcarcel, Anal. Chim. Acta, 1985, 225, 443.
T. Aoki, Y. Fujimura, Y. Oka, and K. Fujie, Anal. Chim. Acta, 1993, 284, 167.
T. Kuwaki, M. Akiba, M. Oshima, and S. Motomizu, Bunseki Kagaku, 1987, 36, T81.
T. Takayanagi, H. Tanaka, and S. Motomizu, Anal. Sci., 1997, 13, 11.
W. Stumm and J. J. Morgan, “Aquatic Chemistry”, 2nd ed., 1981, Wiley, New York, 149.
W. E. Van der Linden, Anal. Chim. Acta, 1983, 155, 273.
R. M. Carlson, Anal. Chem., 1978, 50, 1528.
K. Higuchi, A. Inoue, T. Tsuboi, and S. Motomizu, Bunseki Kagaku, 1999, 48, 253.
L. Ma, M. Oshima, T. Takayanagi, and S. Motomizu, J. Flow Injection Anal., 2000, 17, 188.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Oshima, M., Wei, Y., Yamamoto, M. et al. Highly Sensitive Determination Method for Total Carbonate in Water Samples by Flow Injection Analysis Coupled with Gas-Diffusion Separation. ANAL. SCI. 17, 1285–1290 (2001). https://doi.org/10.2116/analsci.17.1285
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2116/analsci.17.1285