Abstract
Objectives
Estimate HTLV-1/2 (human T-cell lymphotropic viruses) prevalence in Canadian blood donors and the association of demographic variables with infection and their corresponding risk factors.
Methods
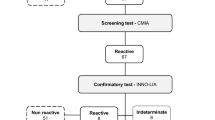
First-time blood donors in all Canadian provinces (except Quebec) from 1990 to 2022 were included. Blood samples were tested for HTLV-1/2 by enzyme-linked immunoassay, confirmed by Western blot. Multivariable logistic regression with year, age group, sex, region, neighbourhood material deprivation, and ethnocultural composition indices predicted HTLV-1/2. Since 2005, all HTLV-1/2-positive donors (cases) were invited to participate in a risk factor interview, and 4 non-positive donors (controls per case) were matched for age, sex, and region. Case–control predictors of HTLV-1/2 were analyzed using logistic regression.
Results
There were 3,085,554 first-time donors from 1990 to 2022. HTLV-1/2 prevalence remained low (12 per 100,000 in 2022, 95% CI 6.4–23.5). The odds ratios predicting HTLV-1/2 were higher in females (2.0, 95% CI 1.5–2.6), older age groups (50 + ; 6.3, 95% CI 4.3–9.2), British Columbia and Ontario, those materially deprived (1.9, 95% CI 1.2–2.9), and those in ethnocultural neighbourhoods (7.5, 95% CI 3.2–17.3). Most HTLV-1/2 in Ontario was HTLV-1, whereas in British Columbia half were HTLV-2. Forty-three of 149 (28.8%) cases and 172 of 413 (41.6%) controls completed an interview. The strongest predictor of HTLV-1/2 in case–control analysis was birth in a high-prevalence country (OR 39.8, 95% CI 7.8–204.3) but about 50% of HTLV-1 and 90% of HTLV-2 were Canadian-born.
Conclusion
HTLV-1/2 prevalence is low in blood donors. High-prevalence country of birth accounts for about half of HTLV-1; HTLV-2 positives are usually Canadian-born. HTLV-1/2 transmission likely occurs overseas and within Canada.
Résumé
Objectifs
Estimer la prévalence des sous-types du virus T-lymphotrope humain (HTLV-1 et HTLV-2) dans le sang des donneurs de sang canadiens, et évaluer le lien avec des variables démographiques et des facteurs de risque donnés.
Méthodes
Cette étude a porté sur toutes les personnes ayant fait leur premier don entre 1990 et 2022 au Canada, sauf au Québec. Les échantillons de sang ont été soumis à un test immunoenzymatique, puis à un test Western Blot de confirmation. Les données ont été analysées au moyen de la régression logistique en utilisant comme indices l’année, la tranche d’âge, le sexe, la région, le quartier, la privation matérielle et la composition ethnoculturelle. Depuis 2005, tous les donneurs positifs au HTLV-1/2 (cas) ont été conviés à un entretien ayant pour but de déterminer leurs facteurs de risque, et quatre donneurs négatifs (cas-témoins) ont été appariés à chaque cas en fonction de l’âge, du sexe et de la région. Les facteurs de prédiction d’infection au HTLV-1/2 des cas-témoins ont été analysés au moyen de la régression logistique.
Résultats
Entre 1990 et 2022, le nombre de primodonneurs s’élevait à 3 085 554. La prévalence du HTLV-1/2 est demeurée faible (12,2 sur 100 000 en 2022, IC 95%: 6,4–23,5). Le rapport de cotes était plus élevé chez les femmes (2,0, IC 95% 1,5–2,6), chez les personnes de plus de 50 ans (6,3, IC 95% 4,3–9,2), en Colombie-Britannique et en Ontario, chez les personnes touchées par la privation matérielle (1,9, IC 95% 1,2–2,9) et chez les personnes vivant dans des quartiers ethnoculturels (7,5, IC 95% 3,2–17,3). La plupart des cas de HTLV-1/2 rencontrés en Ontario concernaient le HTLV-1, tandis qu’en Colombie-Britannique, la moitié des cas concernait le HTLV-2. Quarante-trois cas sur 149 (28,8 %) et 172 cas-témoins sur 413 (41,6 %) ont passé l’entretien. L’analyse des cas-témoins a révélé que le facteur de prédiction le plus important d’infection au HTLV-1/2 était le fait d’être né dans un pays à forte prévalence (RC 39,8, IC 95% 7,8–204,3); toutefois environ 50 % des cas-témoins de HTLV-1 et 90 % des cas témoins de HTLV-2 étaient nés au Canada.
Conclusion
La prévalence du HTLV-1/2 est faible dans le sang des donneurs de sang. Pays de naissance à forte prévalence représente à peu près la moitié des cas de HTLV-1; les donneurs positifs au HTLV-2 la plupart du temps sont nés au Canada. La transmission du HTLV-1/2 survient probablement outre-mer et au Canada.


Similar content being viewed by others
Data availability
Summary data can be obtained by contacting the corresponding author. De-identified individual data can be made available subject to research ethics approval, internal review, and legal contracts.
Code availability
Not applicable.
References
Amar, L., Le, M., Ghazawi, F. M., Rahme, E., Segal, A., Netchiporouk, E., et al. (2019). Prevalence of human T cell lymphotropic virus 1 infection in Canada. Current Oncology, 26(1), e1-15.
Andonov, A., Coulthart, M. B., Perez-Losada, M., Crandall, K. A., Posada, D., Padmore, R., et al. (2012). Insights into origins of Human T-cell Lymphotropic Virus Type 1 based on new strains from aboriginal people of Canada. Infect Genetics Evol, 12(8), 1822–1830.
Brant, L. J., Cawley, C., Davison, K. L., & Taylor, G. P. (2011). Recruiting individuals into the HTLV cohort study in the United Kingdom: Clinical findings and challenges in the first six years, 2003 to 2009. Eurosurveillance, 16(46), 200017.
Canadian Blood Services. (2023). ABC’s of eligibility to donate blood, platelets and plasma. https://www.blood.ca/en/blood/am-i-eligible-donate-blood/abcs-eligibility. Accessed 20 Feb 2024.
European Centre for Disease Prevention and Control. (2015). Geographical distribution of areas with a high prevalence of HTLV-1 infection. https://www.geographical-distribution-areas-high-prevalence-HTLV1.pdf (europa.eu). Accessed 2 Nov 2023.
Gessain, A., Ramassamy, J. L., Alfonso, P. V., & Cassar, O. (2023). Geographic distribution, clinical epidemiology and genetic diversity of the human oncogenic retrovirus HTLV-1 in Africa, the world’s largest endemic area. Frontiers in Immunology, 14, 1043600.
Gessain, A., & Casser, O. (2012). Epidemiological aspects and world distribution of HTLV-1 infection. Frontiers in Microbiology, 3, 388.
Government of Canada. (2023). Medical exams – Immigration. https://www.canada.ca/en/immigration-refugees-citizenship/services/application/medical-police/medical-exams.html. Accessed 26 Jan 2024.
Hedayati-Moghaddam, M. R., Esfehani, R. J., Hajj, H. E., & Bazarbachi, A. (2022). Updates in the epidemiology of the human T-cell leukemia virus type 1 infection in countries of the Eastern Mediterranean Regional Office of the World Health Organization with special emphasis on the situation in Iran. Viruses, 14(4), 664.
Ishak, R., Ishak, M. O. G., Abreu, I. N., Machado, L. F. A., Lima, S. S., Queiroz, M. A. F., et al. (2023). Long-term prevalence follow-up (1967–2022) of HTLV-2 among vulnerable indigenous populations in the Amazon region of Brazil. Frontiers in Microbiology, 14, 1217134.
Iwanaga, M. (2020). Epidemiology of HTLV-1 infection and ATL in Japan. An Update. Frontiers in Microbiology, 11, 1124.
Lee, Y. H., Lu, C. T., Feng, I. J., & Hung, C. M. (2023). Trend in seroprevalence of HTLV-1/2 in Taiwanese blood donors – A 10 year follow-up. Transfusion Medicine, 33(4), 320–328.
Martin, J. D., Mathias, R. G., Sarin, C., & Byrne, S. E. (2002). Human T-lymphotropic virus type I and II infections in First Nations alcohol and drug treatment centres in British Columbia, Canada, 1992–2000. International Journal of Circumpolar Health, 61(2), 98–103.
Mendoza, C., Rando, A., Miro, E., Pena, M. J., Rodriguez-Avial, I., Ortega, D., et al. (2023). Adult T-cell leukemia/lymphoma in HTLV-1 non-endemic regions. Journal of Clinical Virology, 167, 105578.
Murphy, E. L. (2016). Infection with human T-lymphotropic virus types-1 and -2 (HTLV-1 and -2): Implications for blood transfusion safety. Transfusion clinique et biologique, 23(1), 13–19.
Murphy, E.L. (2024). HTLV-1 and blood donation. British Journal of Haematology, 204(1), 29-30.
Murphy, E. L., Watanabe, K., Nass, C. C., Ownby, H., Williams, A., & Nemo, G. (1999). Evidence among blood donors for a 30-year-old epidemic of human T lymphocyte virus type II infection in the United States. The Journal of Infectious Disease, 180(6), 1777–1783.
O’Brien, S. F., Goldman, M., Scalia, V., Yi, Q. L., Fan, W., Xi, G., et al. (2013). The epidemiology of human T-cell lymphotropic virus types I and II in Canadian blood donors. Transfusion Medicine, 23(5), 358–366.
O’Brien, S. F., Yi, Q. L., Goldman, M., Gregoire, Y., & Delage, G. (2018). Human T-cell lymphotropic virus: A simulation model to estimate residual risk with universal leukoreduction and testing strategies in Canada. Vox Sanguinis, 113(8), 750–759.
Orland, J. R., Wang, B., Wright, D. J., Nass, C. C., Garratty, G., Smith, J. W., et al. (2004). Increased mortality associated with HTLV-II infection in blood donors: A prospective cohort study. Retrovirology, 1, 4.
Pampalon, R., Hamel, D., Gamache, P., Philibert, M. D., Raymond, G., & Simpson, A. (2021). An area-based material and social deprivation index for public health in Quebec and Canada. Canadian Journal of Public Health, 103(8 Suppl 2), 17–22.
Patriquin, G., Hatchette, J. E., & Hatchette, T. F. (2020). Canadian physicians’ knowledge, attitudes, and beliefs about the risk of HTLV infection in solid organ transplantation. Journal of the Association of Medical Microbiology and Infectious Disease Canada, 5(3), 124–126.
Peters, A. A., Coulthart, M. B., Oger, J. J., Waters, D. J., Crandall, K. A., Baumgartner, A. A., et al. (2000). HTLV type I/II in British Columbia Amerindians: A seroprevalence study and sequence characterization of an HTLV type IIa isolate. AIDS Research and Human Retroviruses, 16(9), 883–892.
Picard, F. J., Coulthart, M. B., Oger, J., King, E. E., Kim, S., Arp, J., et al. (1995). Human T-lymphotropic virus type 1 in coastal natives of British Columbia: Phylogenetic affinities and possible origins. Journal of Virology, 69(11), 7248–7256.
Piron, M., Salvador, F., Caballero, E., Sanchez-Montalva, A., Bes, M., Casamitjana, N., et al. (2022). HTLV-1/2 infection in blood donors from a non-endemic area (Catalonia, Spain) between 2008 and 2017: A 10-year experience. Viruses, 14(9), 1975.
Prinsze, F. J., & Zaaijer, H. L. (2012). The outcome of donor screening for human T-cell lymphotropic virus infection in the Netherlands. Vox Sanguinis, 102, 198–203.
Rosadas, C., Harvala, H., Davison, K., & Taylor, G. P. (2023). HTLV-1 screening of blood donations: We are systematically missing opportunities. British Journal of Haematology, 32(2), 1220–1223.
Schreiber, G. B., Murphy, E. L., Horton, J. A., Wright, D. J., Garfein, R., Chien, H. C., et al. (1997). Risk factors for human T-cell lymphotropic virus types I and II (HTLV-I and -II). In blood donors: The Retrovirus Epidemiology Donor Study. Journal of Acquired Immunodeficiency Syndrome Human Retrovirology, 14(3), 263–271.
Sobol, I., Palacios, C., Osborne, G., Hildes, J., MacDonald, W., Harty, A., et al. (2007). Initial management of an outbreak of the HTLV-1 virus in Nunavut, Canada. Alaska Medicine, 49(2 Suppl), 204–206.
Sonoda, S., Li, H. C., & Tajima, K. (2011). Ethnoepidemiology of HTLV-1 related diseases: Ethnic determinants of HTLV-1 susceptibility and its worldwide dispersal. Cancer Science, 102(2), 295–301.
Statistics Canada. (2019). Canadian Index of Multiple Deprivation: Dataset and User Guide. https://www150.statcan.gc.ca/n1/en/catalogue/45200001 Accessed 15 Sep 2023.
Statistics Canada. (2022). Immigration. https://www150.statcan.gc.ca/n1/pub/12-581-x/2022001/sec2-eng.htm. Accessed 2 Nov 2023.
World Health Organization. (2021). Global status on blood safety and availability 2021. https://iris.who.int/bitstream/handle/10665/356165/9789240051683-eng.pdf?sequence=1. Accessed 2 Nov 2023.
Xie, J., Ge, G., Zhang, Y., Lin, Y., Ni, H., Zhang, J., et al. (2015). The prevalence of human T-lymphotropic virus infection in blood donors in Southeast China, 2004–2013. PLoS Neglected Tropical Diseases, 9(4), e0003685.
Funding
All work was funded internally by Canadian Blood Services.
Author information
Authors and Affiliations
Contributions
SFO, SJD, and MG conceived the study. LO collected data and LO, WF, and BE conducted the analysis. SFO drafted the manuscript and SJD conducted review and editing. All authors provided critical review and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
All procedures performed in the case–control study involving human participants were in accordance with the ethical standards of the Canadian Blood Services Research Ethics Board (REB 2018.040) and with the 1964 Helsinki Declaration and its later amendments of comparable ethical standards. The surveillance data analysis was deemed exempt by the Canadian Blood Services Research Ethics Board.
Consent to participate
Informed consent was obtained from individual participants in the case–control study. All blood donors were informed that their blood would be tested for infectious markers including HTLV as part of the consent to donate process and provided informed consent.
Consent for publication
Not applicable.
Conflict of interest
SJD has received laboratory supplies from Abbott Pharmaceuticals and consulting fees from Roche.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
See Table 4, Table 5, Table 6 and Table 7
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
O’Brien, S.F., Ehsani-Moghaddam, B., Goldman, M. et al. Prevalence of human T-cell lymphotropic virus-1/2 in Canada over 33 years: A unique contribution of blood donors to public health surveillance. Can J Public Health (2024). https://doi.org/10.17269/s41997-024-00886-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.17269/s41997-024-00886-6