Abstract
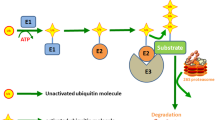
Translationally controlled tumor protein (TCTP) is a highly conserved multifunctional protein localized in the cytoplasm and nucleus of eukaryotic cells. It is secreted through exosomes and its degradation is associated with the ubiquitin-proteasome system (UPS), heat shock protein 27 (Hsp27), and chaperone-mediated autophagy (CMA). Its structure contains three α-helices and eleven β-strands, and features a helical hairpin as its hallmark. TCTP shows a remarkable similarity to the methionine-R-sulfoxide reductase B (MsrB) and mammalian suppressor of Sec4 (Mss4/Dss4) protein families, which exerts guanine nucleotide exchange factor (GEF) activity on small guanosine triphosphatase (GTPase) proteins, suggesting that some functions of TCTP may at least depend on its GEF action. Indeed, TCTP exerts GEF activity on Ras homolog enriched in brain (Rheb) to boost the growth and proliferation of Drosophila cells. TCTP also enhances the expression of cell division control protein 42 homolog (Cdc42) to promote cancer cell invasion and migration. Moreover, TCTP regulates cytoskeleton organization by interacting with actin microfilament (MF) and microtubule (MT) proteins and inducing the epithelial-mesenchymal transition (EMT) process. In essence, TCTP promotes cancer cell movement. It is usually highly expressed in cancerous tissues and thus reduces patient survival; meanwhile, drugs can target TCTP to reduce this effect. In this review, we summarize the mechanisms of TCTP in promoting cancer invasion and migration, and describe the current inhibitory strategy to target TCTP in cancerous diseases.
概要
翻译控制肿瘤蛋白(TCTP)定位于真核细胞的细胞质和细胞核, 是一种高度保守的多功能蛋白。它以外泌体的方式分泌, 通过泛素-蛋白酶体系统(UPS)、热休克蛋白27(Hsp27)和分子伴侣介导的自噬(CMA)发生降解。TCTP的结构包含3个α-螺旋和11个β-链, 并以螺旋发夹作为其结构特征。TCTP与蛋氨酸-R-亚砜还原酶B(MsrB)和哺乳动物Sec4抑制蛋白家族(Mss4/Dss4)的结构有很强的相似性, 这些蛋白家族对小分子GTPase发挥鸟嘌呤核苷酸交换因子(GEF)的活性, 表明TCTP的某些功能可能依赖于GEF的活性。TCTP对Rheb发挥GEF活性以促进果蝇细胞的生长和增殖。TCTP增强细胞分裂控制蛋白42(Cdc42)的表达从而促进癌细胞的侵袭和迁移。此外, TCTP通过与肌动蛋白微丝(MF)和微管(MT)蛋白结合来调控细胞骨架重排, 并参与诱导上皮-间充质转化(EMT)的过程。因此, TCTP促进癌细胞运动。TCTP在癌组织中通常高表达, 这减少了患者的存活率, 然而药物能靶向TCTP会降低其促癌作用。本文综述了TCTP促进癌细胞侵袭和迁移的机制, 并介绍了目前在癌症中抑制TCTP的策略。
Similar content being viewed by others
References
Acunzo J, Baylot V, So A, et al., 2014. TCTP as therapeutic target in cancers. Cancer Treat Rev, 40(6):760–769. https://doi.org/10.1016/j.ctrv.2014.02.007
Alfonso P, Dolado I, Swat A, et al., 2006. Proteomic analysis of p38α mitogen-activated protein kinase regulated changes in membrane fractions of RAS-transformed fibroblasts. Proteomics, 6(S1):S262–S271. https://doi.org/10.1002/pmic.200500350
Amson R, Pece S, Lespagnol A, et al., 2012. Reciprocal repression between P53 and TCTP. Nat Med, 18(1):91–99. https://doi.org/10.1038/nm.2546
Amson R, Karp JE, Telerman A, 2013a. Lessons from tumor reversion for cancer treatment. Curr Opin Oncol, 25(1): 59–65. https://doi.org/10.1097/CCO.0b013e32835b7d21
Amson R, Pece S, Marine JC, et al., 2013b. TPT1/TCTP-regulated pathways in phenotypic reprogramming. Trends Cell Biol, 23(1):37–46. https://doi.org/10.1016/j.tcb.2012.10.002
Amson R, Auclair C, André F, et al., 2017. Targeting TCTP with sertraline and thioridazine in cancer treatment. In: Telerman A, Amson R (Eds.), TCTP/tpt1-Remodeling Signaling from Stem Cell to Disease. Springer, Cham, p.283–290. https://doi.org/10.1007/978-3-319-67591-6_15
Amzallag N, Passer BJ, Allanic D, et al., 2004. TSAP6 facilitates the secretion of translationally controlled tumor protein/histamine-releasing factor via a nonclassical pathway. J Biol Chem, 279(44):46104–46112. https://doi.org/10.1074/jbc.M404850200
Arcuri F, Papa S, Carducci A, et al., 2004. Translationally controlled tumor protein (TCTP) in the human prostate and prostate cancer cells: expression, distribution, and calcium binding activity. Prostate, 60(2): 130–140. https://doi.org/10.1002/pros.20054
Bae SY, Kim HJ, Lee KJ, et al., 2015. Translationally controlled tumor protein induces epithelial to mesenchymal transition and promotes cell migration, invasion and metastasis. Sci Rep, 5:8061. https://doi.org/10.1038/srep08061
Baylot V, Katsogiannou M, Andrieu C, et al., 2012. Targeting TCTP as a new therapeutic strategy in castration-resistant prostate cancer. Mol Ther, 20(12):2244–2256. https://doi.org/10.1038/mt.2012.155
Baylot V, Karaki S, Rocchi P, 2017. TCTP has a crucial role in the different stages of prostate cancer malignant progression. In: Telerman A, Amson R (Eds.), TCTP/tpt1-Remodeling Signaling from Stem Cell to Disease. Springer, Cham, p.255–261. https://doi.org/10.1007/978-3-319-67591-6_13
Bazile F, Pascal A, Arnal I, et al., 2009. Complex relationship between TCTP, microtubules and actin microfilaments regulates cell shape in normal and cancer cells. Carcinogenesis, 30(4):555–565. https://doi.org/10.1093/carcin/bgp022
Bell NJ, Hunt RH, 1992. Role of gastric acid suppression in the treatment of gastro-oesophageal reflux disease. Gut, 33(1):118–124. https://doi.org/10.1136/gut.33.1.118
Bommer UA, Thiele BJ, 2004. The translationally controlled tumour protein (TCTP). Int J Biochem Cell Biol, 36(3): 379–385. https://doi.org/10.1016/s1357-2725(03)00213-9
Bonhoure A, Vallentin A, Martin M, et al., 2017. Acetylation of translationally controlled tumor protein promotes its degradation through chaperone-mediated autophagy. Eur J Cell Biol, 96(2):83–98. https://doi.org/10.1016/j.ejcb.2016.12.002
Brioudes F, Thierry AM, Chambrier P, et al., 2010. Translationally controlled tumor protein is a conserved mitotic growth integrator in animals and plants. Proc Natl Acad Sci USA, 107(37):16384–16389. https://doi.org/10.1073/pnas.1007926107
Cans C, Passer BJ, Shalak V, et al., 2003. Translationally controlled tumor protein acts as a guanine nucleotide dissociation inhibitor on the translation elongation factor eEF1A. Proc Natl Acad Sci USA, 100(24):13892–13897. https://doi.org/10.1073/pnas.2335950100
Chaffer CL, Weinberg RA, 2011. A perspective on cancer cell metastasis. Science, 331(6024):1559–1564. https://doi.org/10.1126/science.1203543
Chan THM, Chen LL, Guan XY, 2012a. Role of translationally controlled tumor protein in cancer progression. Biochem Res Int, 2012:369384. https://doi.org/10.1155/2012/369384
Chan THM, Chen LL, Liu M, et al., 2012b. Translationally controlled tumor protein induces mitotic defects and chromosome missegregation in hepatocellular carcinoma development. Hepatology, 55(2):491–505. https://doi.org/10.1002/hep.24709
Chen C, Deng Y, Hua MH, et al., 2015. Expression and clinical role of TCTP in epithelial ovarian cancer. J Mol Histol, 46(2):145–156. https://doi.org/10.1007/s10735-014-9607-y
Chen YL, Yan MY, Chien SY, et al., 2013. Sann-Joong-Kuey-Jian-Tang inhibits hepatocellular carcinoma Hep-G2 cell proliferation by increasing TNF-α, Caspase-8, Caspase-3 and Bax but by decreasing TCTP and Mcl-1 expression in vitro. Mol Med Rep, 7(5): 1487–1493. https://doi.org/10.3892/mmr.2013.1381
Cheng CY, Lin YH, Su CC, 2010. Curcumin inhibits the proliferation of human hepatocellular carcinoma J5 cells by inducing endoplasmic reticulum stress and mitochondrial dysfunction. Int J Mol Med, 26(5):673–678. https://doi.org/10.3892/ijmm_00000513
Chinnapaka S, Bakthavachalam V, Munirathinam G, 2020. Repurposing antidepressant sertraline as a pharmacological drug to target prostate cancer stem cells: dual activation of apoptosis and autophagy signaling by deregulating redox balance. Am J Cancer Res, 10(7):2043–2065.
Cho JH, Park S, Kim S, et al., 2022. RKIP induction promotes tumor differentiation via SOX2 degradation in NF2-deficient conditions. Mol Cancer Res, 20(3):412–424. https://doi.org/10.1158/1541-7786.MCR-21-0373
Choi S, Min HJ, Kim M, et al., 2009. Proton pump inhibitors exert anti-allergic effects by reducing TCTP secretion. PLoS ONE, 4(6):e5732. https://doi.org/10.1371/journal.pone.0005732
Chu ZH, Liu L, Zheng CX, et al., 2011. Proteomic analysis identifies translationally controlled tumor protein as a mediator of phosphatase of regenerating liver-3-promoted proliferation, migration and invasion in human colon cancer cells. Chin Med J, 124(22):3778–3785. https://doi.org/10.3760/cmaj.issn.0366-6999.2011.22.032
D’Amico S, Krasnowska EK, Manni I, et al., 2020. DHA affects microtubule dynamics through reduction of phospho-TCTP levels and enhances the antiproliferative effect of T-DM1 in trastuzumab-resistant HER2-positive breast cancer cell lines. Cells, 9(5): 1260. https://doi.org/10.3390/cells9051260
Dong XC, Yang B, Li YJ, et al., 2009. Molecular basis of the acceleration of the GDP-GTP exchange of human Ras homolog enriched in brain by human translationally controlled tumor protein. J Biol Chem, 284(35):23754–23764. https://doi.org/10.1074/jbc.M109.012823
Fischer N, Seo EJ, Klinger A, et al., 2021. AMG900 as novel inhibitor of the translationally controlled tumor protein. Chem Biol Interact, 334:109349. https://doi.org/10.1016/j.cbi.2020.109349
Fujita T, Felix K, Pinkaew D, et al., 2008. Human fortilin is a molecular target of dihydroartemisinin. FEBS Lett, 582(7): 1055–1060. https://doi.org/10.1016/j.febslet2008.02.055
Gao JY, Ma FJ, Wang XJ, et al., 2020. Combination of dihydroartemisinin and resveratrol effectively inhibits cancer cell migration via regulation of the DLC1/TCTP/Cdc42 pathway. Food Funct, 11(11):9573–9584. https://doi.org/10.1039/d0fo00996b
Gross B, Gaestel M, Böhm H, et al., 1989. cDNA sequence coding for a translationally controlled human tumor protein. Nucleic Acids Res, 17(20):8367. https://doi.org/10.1093/nar/17.20.8367
Haghighat NG, Ruben L, 1992. Purification of novel calcium binding proteins from Trypanosoma brucei: properties of 22-, 24- and 38-kilodalton proteins. Mol Biochem Parasitol, 51(1):99–110. https://doi.org/10.1016/0166-6851(92)90205-x
Ho KW, Lambert WS, Calkins DJ, 2014. Activation of the TRPV1 cation channel contributes to stress-induced astrocyte migration. GLIA, 62(9):1435–1451. https://doi.org/10.1002/glia.22691
Howe AK, 2004. Regulation of actin-based cell migration by cAMP/PKA. Biochim Biophys Acta (BBA)-Mol Cell Res, 1692(2–3):159–174. https://doi.org/10.1016/j.bbamcr.2004.03.005
Hsu YC, Chern JJ, Cai Y, et al., 2007. Drosophila TCTP is essential for growth and proliferation through regulation of dRheb GTPase. Nature, 445(7129):785–788. https://doi.org/10.1038/nature05528
Huang CY, Chiu TL, Kuo SJ, et al., 2013. Tanshinone IIA inhibits the growth of pancreatic cancer BxPC-3 cells by decreasing protein expression of TCTP, MCL-1 and Bcl-xL. Mol Med Rep, 7(3): 1045–1049. https://doi.org/10.3892/mmr.2013.1290
Huang HY, Li X, Hu GH, et al., 2015. Poly(ADP-ribose) glycohydrolase silencing down-regulates TCTP and Cofilin-1 associated with metastasis in benzo(a)pyrene carcinogenesis. Am J Cancer Res, 5(1): 155–167.
Huang M, Geng Y, Deng Q, et al., 2018. Translationally controlled tumor protein affects colorectal cancer metastasis through the high mobility group box 1-dependent pathway. Int J Oncol, 53(4):1481–1492. https://doi.org/10.3892/ijo.2018.4502
Ishida H, Jensen KV, Woodman AG, et al., 2017. The calcium-dependent switch helix of L-plastin regulates actin bundling. Sci Rep, 7:40662. https://doi.org/10.1038/srep40662
Jaglarz MK, Bazile F, Laskowska K, et al., 2012. Association of TCTP with centrosome and microtubules. Biochem Res Int, 2012:541906. https://doi.org/10.1155/2012/541906
Jin H, Zhang XX, Su J, et al., 2015. RNA interference-mediated knockdown of translationally controlled tumor protein induces apoptosis, and inhibits growth and invasion in glioma cells. MolMedRep, 12(5):6617–6625. https://doi.org/10.3892/mmr.2015.4280
Kim M, Jung Y, Lee K, et al., 2000. Identification of the calcium binding sites in translationally controlled tumor protein. Arch Pharm Res, 23(6):633–636. https://doi.org/10.1007/BF02975253
Kloc M, Tejpal N, Sidhu J, et al., 2012. Inverse relationship between TCTP/RhoA and p53/cyclin A/actin expression in ovarian cancer cells. Folia Histochem Cytobiol, 50(3): 358–367. https://doi.org/10.5603/19745
Koziol MJ, Gurdon JB, 2012. TCTP in development and cancer. Biochem Res Int, 2012:105203. https://doi.org/10.1155/2012/105203
Lee JS, Jang EH, Woo HA, et al., 2020. Regulation of autophagy is a novel tumorigenesis-related activity of multifunctional translationally controlled tumor protein. Cells, 9(1):257. https://doi.org/10.3390/cells9010257
Li F, Zhang D, Fujise K, 2001. Characterization of fortilin, a novel antiapoptotic protein. J Biol Chem, 276(50):47542–47549. https://doi.org/10.1074/jbc.M108954200
Liu LZ, Wang MH, Xin QH, et al., 2020. The permissive role of TCTP in PM2.5/NNK-induced epithelial-mesenchymal transition in lung cells. J Transl Med, 18:66. https://doi.org/10.1186/s12967-020-02256-5
Liu TY, Zhou LL, Xiao Y, et al., 2022. BRAF inhibitors reprogram cancer-associated fibroblasts to drive matrix remodeling and therapeutic escape in melanoma. Cancer Res, 82(3):419–432. https://doi.org/10.1158/0008-5472.CAN-21-0614
Lowther WT, Weissbach H, Etienne F, et al., 2002. The mirrored methionine sulfoxide reductases of Neisseria gonorrhoeae pilB. Nat Struct Biol, 9(5):348–352. https://doi.org/10.1038/nsb783
Lucibello M, Adanti S, Antelmi E, et al., 2015. Phospho-TCTP as a therapeutic target of Dihydroartemisinin for aggressive breast cancer cells. Oncotarget, 6(7):5275–5291. https://doi.org/10.18632/oncotarget.2971
Lupas AN, Zhu HB, Korycinski M, 2015. The thalidomidebinding domain of cereblon defines the CULT domain family and is a new member of the β-tent fold. PLoS Comput Biol, 11(1):e1004023. https://doi.org/10.1371/journal.pcbi.1004023
Ma Q, Geng Y, Xu WW, et al., 2010. The role of translationally controlled tumor protein in tumor growth and metastasis of colon adenocarcinoma cells. J Proteome Res, 9(1):40–49. https://doi.org/10.1021/pr9001367
MacDonald SM, Rafnar T, Langdon J, et al., 1995. Molecular identification of an IgE-dependent histamine-releasing factor. Science, 269(5224):688–690. https://doi.org/10.1126/science.7542803
Mertens AE, Roovers RC, Collard JG, 2003. Regulation of Tiam1-Rac signalling. FEBS Lett, 546(1):11–16. https://doi.org/10.1016/s0014-5793(03)00435-6
Mishra DK, Srivastava P, Sharma A, et al., 2018. Translationally controlled tumor protein (TCTP) is required for TGF-β1 induced epithelial to mesenchymal transition and influences cytoskeletal reorganization. Biochim Biophys Acta (BBA)-Mol Cell Res, 1865(1):67–75. https://doi.org/10.1016/j.bbamcr.2017.09.014
Nagano-Ito M, Ichikawa S, 2012. Biological effects of mammalian translationally controlled tumor protein (TCTP) on cell death, proliferation, and tumorigenesis. Biochem Res Int, 2012:204960. https://doi.org/10.1155/2012/204960
Pacher P, Kecskemeti V, 2004. Cardiovascular side effects of new antidepressants and antipsychotics: new drugs, old concerns? Curr Pharm Des, 10(20):2463–2475. https://doi.org/10.2174/1381612043383872
Phanthaphol N, Techasen A, Loilome W, et al., 2017. Upregulation of TCTP is associated with cholangiocarcinoma progression and metastasis. Oncol Lett, 14(5):5973–5979. https://doi.org/10.3892/ol.2017.6985
Proietti S, Cucina A, Pensotti A, et al., 2019. Active fraction from embryo fish extracts induces reversion of the malignant invasive phenotype in breast cancer through down-regulation of TCTP and modulation of E-cadherin/β-catenin pathway. Int J Mol Sci, 20(9):2151. https://doi.org/10.3390/ijms20092151
Raghavamenon AC, Muyiwa AF, Davis LK, et al., 2011. Dihydroartemisinin induces caspase-8-dependent apoptosis in murine GT1-7 hypothalamic neurons. Toxicol Mech Methods, 21(5):367–373. https://doi.org/10.3109/15376516.2011.552534
Ramani P, Nash R, Sowa-Avugrah E, et al., 2015. High levels of polo-like kinase 1 and phosphorylated translationally controlled tumor protein indicate poor prognosis in neuroblastomas. J Neurooncol, 125(1):103–111. https://doi.org/10.1007/s11060-015-1900-4
Rehmann H, Brüning M, Berghaus C, et al., 2008. Biochemical characterisation of TCTP questions its function as a guanine nucleotide exchange factor for Rheb. FEBS Lett, 582(20):3005–3010. https://doi.org/10.1016/j.febslet.2008.07.057
Ren JB, Mao XX, Chen MH, et al., 2015. TCTP expression after rat spinal cord injury: implications for astrocyte proliferation and migration. J Mol Neurosci, 57(3):366–375. https://doi.org/10.1007/s12031-015-0628-0
Rho SB, Lee JH, Park MS, et al., 2011. Anti-apoptotic protein TCTP controls the stability of the tumor suppressor p53. FEBS Lett, 585(1):29–35. https://doi.org/10.1016/j.febslet.2010.11.014
Seo EJ, Efferth T, 2016. Interaction of antihistaminic drugs with human translationally controlled tumor protein (TCTP) as novel approach for differentiation therapy. Oncotarget, 7(13):16818–16839. https://doi.org/10.18632/oncotarget.7605
Su CC, 2014. Tanshinone IIA inhibits human gastric carcinoma AGS cell growth by decreasing BiP, TCTP, Mcl-1 and Bcl-xL and increasing Bax and CHOP protein expression. Int J Mol Med, 34(6): 1661–1668. https://doi.org/10.3892/ijmm.2014.1949
Sun RL, Lu X, Gong L, et al., 2019. TCTP promotes epithelialmesenchymal transition in lung adenocarcinoma. Onco-Targets Ther, 12:1641–1653. https://doi.org/10.2147/OTT.S184555
Susini L, Besse S, Duflaut D, et al., 2008. TCTP protects from apoptotic cell death by antagonizing Bax function. Cell Death Differ, 15(8):1211–1220. https://doi.org/10.1038/cdd.2008.18
Thaw P, Baxter NJ, Hounslow AM, et al., 2001. Structure of TCTP reveals unexpected relationship with guanine nucleotide-free chaperones. Nat Struct Biol, 8(8):701–704. https://doi.org/10.1038/90415
Tsarova K, Yarmola EG, Bubb MR, 2010. Identification of a cofilin-like actin-binding site on translationally controlled tumor protein (TCTP). FEBS Lett, 584(23):4756–4760. https://doi.org/10.1016/j.febslet.2010.10.054
Tuynder M, Susini L, Prieur S, et al., 2002. Biological models and genes of tumor reversion: cellular reprogramming through tpt1/TCTP and SIAH-1. Proc Natl Acad Sci USA, 99(23):14976–14981. https://doi.org/10.1073/pnas.222470799
Tuynder M, Fiucci G, Prieur S, et al., 2004. Translationally controlled tumor protein is a target of tumor reversion. Proc Natl Acad Sci USA, 101(43):15364–15369. https://doi.org/10.1073/pnas.0406776101
van Rheenen J, Condeelis J, Glogauer M, 2009. A common cofilin activity cycle in invasive tumor cells and inflammatory cells. J Cell Sci, 122(3):305–311. https://doi.org/10.1242/jcs.031146
Wandall JH, 1992. Effects of omeprazole on neutrophil chemotaxis, super oxide production, degranulation, and translocation of cytochrome b-245. Gut, 33(5):617–621. https://doi.org/10.1136/gut.33.5.617
Wang LL, Tang YF, Zhao MJ, et al., 2018. Knockdown of translationally controlled tumor protein inhibits growth, migration and invasion of lung cancer cells. Life Sci, 193:292–299. https://doi.org/10.1016/j.lfs.2017.09.039
Wang XM, Fonseca BD, Tang H, et al., 2008. Re-evaluating the roles of proposed modulators of mammalian target of rapamycin complex 1 (mTORC1) signaling. J Biol Chem, 283(45):30482–30492. https://doi.org/10.1074/jbc.M803348200
Wang ZX, Wang Z, Li GH, et al., 2017. CXCL1 from tumorassociated lymphatic endothelial cells drives gastric cancer cell into lymphatic system via activating integrin β1/FAK/AKT signaling. Cancer Lett, 385:28–38. https://doi.org/10.1016/j.canlet.2016.10.043
Wei Y, Redel C, Ahlner A, et al., 2022. The MYC oncoprotein directly interacts with its chromatin cofactor PNUTS to recruit PP1 phosphatase. Nucleic Acids Res, 50(6):3505–3522. https://doi.org/10.1093/nar/gkac138
Xiao B, Chen DX, Luo SH, et al., 2016. Extracellular translationally controlled tumor protein promotes colorectal cancer invasion and metastasis through Cdc42/JNK/MMP9 signaling. Oncotarget, 7(31):50057–50073. https://doi.org/10.18632/oncotarget.10315
Yao Y, Jia XY, Tian HY, et al., 2009. Comparative proteomic analysis of colon cancer cells in response to oxaliplatin treatment. Biochim Biophys Acta (BBA)-Proteins Proteom, 1794(10):1433–1440. https://doi.org/10.1016/j.bbapap.2009.06.005
Yarm FR, 2002. Plk phosphorylation regulates the microtubulestabilizing protein TCTP. Mol Cell Biol, 22(17):6209–6221. https://doi.org/10.1128/MCB.22.17.6209-6221.2002
Yu QL, Zhang B, Zhang YM, et al., 2020. Actin cytoskeletondisrupting and magnetic field-responsive multivalent supramolecular assemblies for efficient cancer therapy. ACS Appl Mater Interfaces, 12(12):13709–13717. https://doi.org/10.1021/acsami.0c01762
Zhang F, Liu B, Wang Z, et al., 2013. A novel regulatory mechanism of Pim-3 kinase stability and its involvement in pancreatic cancer progression. Mol Cancer Res, 11(12): 1508–1520. https://doi.org/10.1158/1541-7786.MCR-13-0389
Zhang F, Ma Q, Xu ZH, et al., 2017. Dihydroartemisinin inhibits TCTP-dependent metastasis in gallbladder cancer. J Exp Clin Cancer Res, 36:68. https://doi.org/10.1186/s13046-017-0531-3
Zhou C, Fan ZS, Zhou ZH, et al., 2022. Discovery of the first-in-class agonist-based SOS1 PROTACs effective in human cancer cells harboring various KRAS mutations. J Med Chem, 65(5):3923–3942. https://doi.org/10.1021/acs.jmedchem.1c01774
Acknowledgments
This work was funded by the National Natural Science Foundation of China (No. 31672377).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Author contributions
Guorong LI and Guiwen YANG outlined the content of the review and made the final corrections for the manuscript. Junying GAO and Yan MA collected the literature and wrote the manuscript. Junying GAO drew the figures. All authors have read and agreed the final manuscript.
Compliance with ethics guidelines
Junying GAO, Yan MA, Guiwen YANG, and Guorong LI declare that they have no conflict of interest.
This article does not contain any studies with human or animal subjects performed by any of the authors.
Rights and permissions
About this article
Cite this article
Gao, J., Ma, Y., Yang, G. et al. Translationally controlled tumor protein: the mediator promoting cancer invasion and migration and its potential clinical prospects. J. Zhejiang Univ. Sci. B 23, 642–654 (2022). https://doi.org/10.1631/jzus.B2100910
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1631/jzus.B2100910