Abstract
Soybean isoflavones have been one of the potential preventive candidates for antitumor research in recent years. In this paper, we first studied the transformation of soybean isoflavones with the homogenized slurry of Ganoderma lucidum. The resultant transformed products (TSI) contained (703.21±4.35) mg/g of genistein, with transformed rates of 96.63% and 87.82% of daidzein and genistein, respectively, and TSI also could enrich the bioactive metabolites of G. lucidum. The antitumor effects of TSI on human colorectal cancer cell line HTL-9, human breast cancer cell line MCF-7, and human immortalized gastric epithelial cell line GES-1 were also studied. The 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide (MTT) assay showed that TSI could dramatically reduce the viability rates of HTL-9 cells and MCF-7 cells without detectable cytotoxicity on GES-1 normal cells when the TSI concentration was lower than 100 μg/ml. With 100 μg/ml of TSI, HTL-9 cells were arrested in the G1 phase, and late-apoptosis was primarily induced, accompanied with partial early-apoptosis. TSI could induce primarily earlyapoptosis by arresting cells in the G1 phase of MCF-7 cells. For HTL-9 cells, Western-blot and reverse-transcriptase polymerase chain reaction (RT-PCR) analysis showed that TSI (100 μg/ml) can up-regulate the expression of Bax, Caspase-3, Caspase-8, and cytochrome c (Cyto-c), indicating that TSI could induce cell apoptosis mainly through the mitochondrial pathway. In addition, the expression of p53 was up-regulated, while the expression of Survivin and nuclear factor κB (NF-κB) was down-regulated. All these results showed that TSI could induce apoptosis of HTL-9 cells by the regulation of multiple apoptosis-related genes.
中文概要
目的
通过灵芝菌生物转化大豆异黄酮,得到富含苷元及灵芝活性成分的多因子转化产物,并研究了转化产物对结直肠癌细胞HTL-9 的体外凋亡诱导,初步探讨转化产物的抗癌活性及机理。
创新点
灵芝是一种珍贵的药用真菌,大豆异黄酮的苷元物质也具有重要的药理活性,本文首次利用灵芝菌液体发酵的匀浆液生物转化大豆异黄酮,所得到的产物中大豆苷元与染料木素转化率高,同时还富集了灵芝菌的活性成分,并对转化产物的抗癌活性及机理进行了初步探讨。
方法
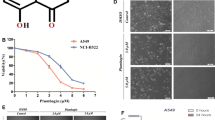
首先利用灵芝菌液体发酵的匀浆体系生物转化大豆异黄酮(图1)。其次,对转化产物的抗癌活性进行研究,主要包括对癌细胞存活率(图2)、细胞凋亡(图3)及细胞周期分布(图4)的影响。最后,利用蛋白质印迹(Western-blot)与逆转录聚合酶链反应(RT-PCR)技术对凋亡相关的基因和蛋白进行检测(图5 和表2),初步探讨转化产物的体外抗癌机理。
结论
本实验结果显示,转化产物中大豆苷元及染料木素的转化率分别为96.63%和87.82%,其中染料木素的含量可达(703.21±4.35) mg/g,同时转化产物中还富含了灵芝菌的活性成分。其次,对转化产物抗癌活性研究发现,其能有效降低HTL-9 细胞的存活率,可通过将细胞阻滞于G1 期而诱导细胞晚期凋亡。此外,转化产物(100 μg/ml)还可明显上调Bax、Caspase-3、Caspase-8、Cyto-c和p53 的表达量,而Survivin 和NF-κB 表达量发生明显下调。结果表明,转化产物主要通过线粒体途径诱导细胞凋亡,但同时还调控多个与凋亡相关的基因。
Similar content being viewed by others
References
Aarnink, A.J.A., Verstegen, M.W.A., 2007. Nutrition, key factor to reduce environmental load from pig production. Livest. Sci., 109(1-3):194–203. http://dx.doi.org/10.1016/j.livsci.2007.01.112
AOAC, 2006. Official Methods of Analysis, 18th Ed. Association of Official Analytical Chemists, Arlington, VA, USA.
Banhazi, T.M., Seedorf, J., Rutley, D.L., et al., 2008. Identification of risk factors for sub-optimal housing conditions in Australian piggeries: Part 2. Airborne pollutants. J. Agric. Saf. Health, 14(1):21–39. http://dx.doi.org/10.13031/2013.24122
Brown, J.A., Cline, T.R., 1974. Urea excretion in the pig: an indicator of protein quality and amino acid requirements. J. Nutr., 104(5):542–545.
Canh, T.T., Sutton, A.L., Aarnink, A.J., et al., 1998. Dietary carbohydrates alter the fecal composition and pH and the ammonia emission from slurry of growing pigs. J. Anim. Sci., 76(7):1887–1895. http://dx.doi.org/10.2527/1998.7671887x
Canibe, N., Jensen, B.B., 2003. Fermented and nonfermented liquid feed to growing pigs: effect on aspects of gastrointestinal ecology and growth performance. J. Anim. Sci., 81(8):2019–2031. http://dx.doi.org/10.2527/2003.8182019x
Chiavegato, M.B., Powers, W., Palumbo, N., 2015. Ammonia and greenhouse gas emissions from housed Holstein steers fed different levels of diet crude protein. J. Anim. Sci., 93(1):395–404. http://dx.doi.org/10.2527/jas.2014-8167
Cho, J.H., Chen, Y.J., Min, B.J., et al., 2008. Effects of reducing dietary crude protein on growth performance, odor gas emission from manure and blood urea nitrogen and IGF-1 concentrations of serum in nursery pigs. Anim. Sci. J., 79(4):453–459. http://dx.doi.org/10.1111/j.1740-0929.2008.00549.x
Dumont, E., Hamon, L., Lagadec, S., et al., 2014. NH3 biofiltration of piggery air. J. Environ. Manage., 140:26–32. http://dx.doi.org/10.1016/j.jenvman.2014.03.008
Griess, P., 1879. Griess reagent: a solution of sulphanilic acid and α-naphthylamine in acetic acid which gives a pink colour on reaction with the solution obtained after decomposition of nitrosyl complexes. Chem. Ber., 12:427 (in German).
Groenestein, C.M., Oosthoek, J., van Faassen, H.G., 1993. Microbial Processes in Deep-Litter Systems for Fattening Pigs and Emission of Ammonia, Nitrous Oxide and Nitric Oxide. EAAP Publication, the Netherlands.
Hamscher, G., Pawelzick, H.T., Sczesny, S., et al., 2003. Antibiotics in dust originating from a pig-fattening farm: a new source of health hazard for farmers? Environ. Health Perspect.,}} 111(13):1590–1594.
Hayes, E.T., Leek, A.B.G., Curran, T.P., et al., 2004. The influence of diet crude protein level on odour and ammonia emissions from finishing pig houses. Bioresour. Technol., 91(3):309–315. http://dx.doi.org/10.1016/S0960-8524(03)00184-6
Hobbs, P.J., Misselbrook, T.H., Pain, B.F., 1997. Characterisation of odorous compounds and emissions from slurries produced from weaner pigs fed dry feed and liquid diets. J. Sci. Food Agric., 73(4):437–445. http://dx.doi.org/10.1002/(SICI)1097-0010(199704)73:4<437::AID-JSFA748<3.0.CO;2-7
Hoff, S.J., Bundy, D.S., Nelson, M.A., et al., 2006. Emissions of ammonia, hydrogen sulfide, and odor before, during, and after slurry removal from a deep-pit swine finisher. J. Air Waste Manag. Assoc., 56(5):581–590. http://dx.doi.org/10.1080/10473289.2006.10464472
Ilea, R.C., 2009. Intensive livestock farming: global trends, increased environmental concerns, and ethical solutions. J. Agric. Environ. Ethics, 22(2):153–167. http://dx.doi.org/10.1007/s10806-008-9136-3
Jones, C.K., DeRouchey, J.M., Nelssen, J.L., et al., 2010. Effects of fermented soybean meal and specialty animal protein sources on nursery pig performance. J. Anim. Sci., 88(5):1725–1732. http://dx.doi.org/10.2527/jas.2009-2110
Kohn, R.A., Dinneen, M.M., Russek-Cohen, E., 2005. Using blood urea nitrogen to predict nitrogen excretion and efficiency of nitrogen utilization in cattle, sheep, goats, horses, pigs and rats. J. Anim. Sci., 83(4):879–889. http://dx.doi.org/10.2527/2005.834879x
Krupa, S.V., 2003. Effects of atmospheric ammonia (NH3) on terrestrial vegetation: a review. Environ. Pollut., 124(2): 179–221. http://dx.doi.org/10.1016/S0269-7491(02)00434-7
Le, P.D., Aarnink, A.J.A., Jongbloed, A.W., et al., 2008. Interactive effects of dietary crude protein and fermentable carbohydrate levels on odour from pig manure. Livest. Sci., 114(1):48–61. http://dx.doi.org/10.1016/j.livsci.2007.04.009
Leonard, R.H., 1963. Quantitative range of Nessler’s reaction with ammonia. Clin. Chem., 9(4):417–422.
Loehr, R.C., Prakasam, T.B.S., Srinath, E.G., et al., 1973. Development and Demonstration of Nutrient Removal from Animal Wastes. Office of Research and Monitoring. Environmental Protection Agency. US Government Printing Office, Washington, DC.
Lynch, M.B., O'Shea, C.J., Sweeney, T., et al., 2008. Effect of crude protein concentration and sugar-beet pulp on nutrient digestibility, nitrogen excretion, intestinal fermentation and manure ammonia and odour emissions from finisher pigs. Animal, 2(3):425–434. http://dx.doi.org/10.1017/S1751731107001267
Min, X., Xiao, J., Kawasaki, K., et al., 2014. Transfer of blood urea nitrogen to cecal microbes and nitrogen retention in mature rabbits are increased by dietary fructooligosaccharides. Anim. Sci. J., 85(6):671–677. http://dx.doi.org/10.1111/asj.12205
Mukherjee, R., Chakraborty, R., Dutta, A., 2016. Role of fermentation in improving nutritional quality of soybean meal—a review. Asian-Aust. J. Anim. Sci., 29(11):1523–1529. http://dx.doi.org/10.5713/ajas.15.0627
Murray, I., Parsons, J.W., Robinson, K., 1975. Interrelationships between nitrogen balance, pH and dissolved oxygen in an oxidation ditch treating farm animal waste. Water Res., 9(1):25–30. http://dx.doi.org/10.1016/0043-1354(75)90148-7
NRC (National Research Council), 2012. Nutrient Requirements of Swine, 11th Ed. Natl. Acad. Press, Washington, DC.
Patráš, P., Nitrayová, S., Brestenský, M., et al., 2012. Effect of dietary fiber and crude protein content in feed on nitrogen retention in pigs. J. Anim. Sci., 90(Suppl. 4):158–160. http://dx.doi.org/10.2527/jas.53837
Pedersen, S., Nonnenmann, M., Rautiainen, R., et al., 2000. Dust in pig buildings. J. Agric. Saf. Health, 6(4):261–274. http://dx.doi.org/10.13031/2013.1909
Portejoie, S., Martinez, J., Guiziou, F., et al., 2003. Effect of covering pig slurry stores on the ammonia emission processes. Bioresour. Technol., 87(3):199–207. http://dx.doi.org/10.1016/S0960-8524(02)00260-2
Renard, J.J., Calidonna, S.E., Henley, M.V., 2004. Fate of ammonia in the atmosphere—a review for applicability to hazardous releases. J. Hazard. Mater., 108(1-2):29–60. http://dx.doi.org/10.1016/j.jhazmat.2004.01.015
Rigolot, C., Espagnol, S., Robin, P., et al., 2010. Modelling of manure production by pigs and NH3, N2O and CH4 emissions. Part II: effect of animal housing, manure storage and treatment practices. Animal, 4(08):1413–1424. http://dx.doi.org/10.1017/S1751731110000509
Shriver, J.A., Carter, S.D., Sutton, A.L., et al., 2003. Effects of adding fiber sources to reduced-crude protein, amino acid-supplemented diets on nitrogen excretion, growth performance, and carcass traits of finishing pigs. J. Anim. Sci., 81(2):492–502. http://dx.doi.org/10.2527/2003.812492x
Suiryanrayna, M.V., Ramana, J.V., 2015. A review of the effects of dietary organic acids fed to swine. J. Anim. Sci. Biotechnol., 6(1):45. http://dx.doi.org/10.1186/s40104-015-0042-z
van Faassen, H.G., van Dijk, H., 1987. Manure as a source of nitrogen and phosphorus in soils. In: van der Meer, H.G., Unwin, R.J., van Dijk, T.A. (Eds.), Animal Manure on Grassland and Fodder Crops. Fertilizer or Waste? Springer Netherlands, p.27–45. http://dx.doi.org/10.1007/978-94-009-3659-1_3
Vogelzang, P.F., van der Gulden, J.W., Folgering, H., et al., 2000. Longitudinal changes in bronchial responsiveness associated with swine confinement dust exposure. Chest, 117(5):1488–1495. http://dx.doi.org/10.1378/chest.117.5.1488
Webb, J., Thorman, R.E., Fernanda-Aller, M., et al., 2014. Emission factors for ammonia and nitrous oxide emissions following immediate manure incorporation on two contrasting soil types. Atmos. Environ., 82:280–287. http://dx.doi.org/10.1016/j.atmosenv.2013.10.043
Wu, L., He, L., Cui, Z., et al., 2015. Effects of reducing dietary protein on the expression of nutrition sensing genes (amino acid transporters) in weaned piglets. J. Zhejiang Univ.-Sci. B (Biomed. & Biotechnol.), 16(6):496–502. http://dx.doi.org/10.1631/jzus.B1400259
Ye, Z., Li, B., Cheng, B., et al., 2007. A concrete slatted floor system for separation of faeces and urine in pig houses. Biosyst. Eng., 98(2):206–214. http://dx.doi.org/10.1016/j.biosystemseng.2007.07.007
Zhou, C., Hu, J., Zhang, B., et al., 2015. Gaseous emissions, growth performance and pork quality of pigs housed in deep-litter system compared to concrete-floor system. Anim. Sci. J., 86(4):422–427. http://dx.doi.org/10.1111/asj.12311
Abbasi, B.H., Stiles, A.R., Saxena, P.K., et al., 2012. Gibberellic acid increases secondary metabolite production in Echinacea purpurea hairy roots. Appl. Biochem. Biotechnol., 168(7):2057–2066. http://dx.doi.org/10.1007/s12010-012-9917-z
Ainsworth, E.A., Gillespie, K.M., 2007. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin-Ciocalteu reagent. Nat. Protoc., 2(4): 875–877. http://dx.doi.org/10.1038/nprot.2007.102
Benzie, I.F.F., Strain, J.J., 1996. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal. Biochem., 239(1):70–76. http://dx.doi.org/10.1006/abio.1996.0292
Cartea, M.E., Velasco, P., 2008. Glucosinolates in Brassica foods: bioavailability in food and significance for human health. Phytochem. Rev., 7(2):213–229. http://dx.doi.org/10.1007/s11101-007-9072-2
Castañeda-Ovando, A., Pacheco-Hernández, M.D.L., Páez-Hernández, M.E., et al., 2009. Chemical studies of anthocyanins: a review. Food Chem., 113(4):859–871. http://dx.doi.org/10.1016/j.foodchem.2008.09.001
Dinkova-Kostova, A.T., Kostov, R.V., 2012. Glucosinolates and isothiocyanates in health and disease. Trends Mol. Med., 18(6):337–347. http://dx.doi.org/10.1016/j.molmed.2012.04.003
Fahey, J.W., Zhang, Y.S., Talalay, P., 1997. Broccoli sprouts: an exceptionally rich source of inducers of enzymes that protect against chemical carcinogens. Proc. Natl. Acad. Sci. USA, 94(19):10367–10372. http://dx.doi.org/10.1073/pnas.94.19.10367
Frerigmann, H., Gigolashvili, T., 2014. MYB34, MYB51, and MYB122 distinctly regulate indolic glucosinolate biosynthesis in Arabidopsis thaliana. Mol. Plant, 7(5):814–828. http://dx.doi.org/10.1093/mp/ssu004
Guo, R., Qian, H., Shen, W., et al., 2013a. BZR1 and BES1 participate in regulation of glucosinolate biosynthesis by brassinosteroids in Arabidopsis. J. Exp. Bot., 64(8):2401–2412. http://dx.doi.org/10.1093/jxb/ert094
Guo, R., Yuan, G., Wang, Q., 2013b. Effect of NaCl treatments on glucosinolate metabolism in broccoli sprouts. J. Zhejiang Univ.-Sci. B (Biomed. & Biotechnol.), 14(2):124–131. http://dx.doi.org/10.1631/jzus.B1200096
Guo, R., Shen, W., Qian, H., et al., 2013c. Jasmonic acid and glucose synergistically modulate the accumulation of glucosinolates in Arabidopsis thaliana. J. Exp. Bot., 64(18):5707–5719. http://dx.doi.org/10.1093/jxb/ert348
Huang, X., He, R., Liao, X., et al., 2014. Effect of exogenous gibberellin on reserve accumulation during the seed filling stage of oilseed rape. Genet. Mol. Res., 13(2):2827–2839. http://dx.doi.org/10.4238/2014.January.22.7
Huseby, S., Koprivova, A., Lee, B.R., et al., 2013. Diurnal and light regulation of sulphur assimilation and glucosinolate biosynthesis in Arabidopsis. J. Exp. Bot., 64(4):1039–1048. http://dx.doi.org/10.1093/jxb/ers378
Jiang, W., Sheng, Q., Jiang, Y., et al., 2004. Effects of 1-methylcyclopropene and gibberellic acid on ripening of Chinese jujube (Zizyphus jujuba M) in relation to quality. J. Sci. Food Agric., 84(1):31–35. http://dx.doi.org/10.1002/jsfa.1594
Kim, H.H., Kwon, D.Y., Uddin, M.R., et al., 2013. Influence of auxins on glucosinolate biosynthesis in hairy root cultures of Broccoli (Brassica oleracea var. italica). Asian J. Chem., 25(11):6099–6101.
Kim, H.J., Chen, F., Wang, X., et al., 2006. Effect of methyl jasmonate on phenolics, isothiocyanate, and metabolic enzymes in radish sprout (Raphanus sativus L.). J. Agric. Food Chem., 54(19):7263–7269. http://dx.doi.org/10.1021/jf060568c
Kumar, G., Tuli, H.S., Mittal, S., et al., 2015. Isothiocyanates: a class of bioactive metabolites with chemopreventive potential. Tumor Biol., 36(6):4005–4016. http://dx.doi.org/10.1007/s13277-015-3391-5
Liang, Z., Ma, Y., Xu, T., et al., 2013. Effects of abscisic acid, gibberellin, ethylene and their interactions on production of phenolic acids in Salvia miltiorrhiza Bunge hairy roots. PLoS ONE, 8(9):e72806. http://dx.doi.org/10.1371/journal.pone.0072806
Loreti, E., Povero, G., Novi, G., et al., 2008. Gibberellins, jasmonate and abscisic acid modulate the sucroseinduced expression of anthocyanin biosynthetic genes in Arabidopsis. New Phytol., 179(4):1004–1016. http://dx.doi.org/10.1111/j.1469-8137.2008.02511.x
Mazumder, A., Dwivedi, A., du Plessis, J., 2016. Sinigrin and its therapeutic benefits. Molecules, 21(4):416. http://dx.doi.org/10.3390/molecules21040416
Miao, H., Wei, J., Zhao, Y., et al., 2013. Glucose signalling positively regulates aliphatic glucosinolate biosynthesis. J. Exp. Bot., 64(4):1097–1109. http://dx.doi.org/10.1093/jxb/ers399
Naeem, N., Ishtiaq, M., Khan, P., et al., 2001. Effect of gibberellic acid on growth and yield of tomato cv. Roma. J. Biol. Sci., 1(6):448–450. http://dx.doi.org/10.3923/jbs.2001.448.450
Park, C.H., Yeo, H.J., Park, Y.J., et al., 2017. Influence of indole-3-acetic acid and gibberellic acid on phenylpropanoid accumulation in common buckwheat (Fagopyrum esculentum Moench) sprouts. Molecules, 22(3):374. http://dx.doi.org/10.3390/molecules22030374
Podsędek, A., 2007. Natural antioxidants and antioxidant capacity of Brassica vegetables: a review. LWT-Food Sci. Technol., 40(1):1–11. http://dx.doi.org/10.1016/j.lwt.2005.07.023
Schweizer, F., Fernándezcalvo, P., Zander, M., et al., 2013. Arabidopsis basic helix-loop-helix transcription factors MYC2, MYC3, and MYC4 regulate glucosinolate biosynthesis, insect performance, and feeding behavior. Plant Cell, 25(8):3117–3132. http://dx.doi.org/10.1105/tpc.113.115139
Sheen, J., 2014. Master regulators in plant glucose signaling networks. J. Plant Biol., 57(2):67–79. http://dx.doi.org/10.1007/s12374-014-0902-7
Sun, B., Liu, N., Zhao, Y., et al., 2011. Variation of glucosinolates in three edible parts of Chinese kale (Brassica alboglabra Bailey) varieties. Food Chem., 124(3):941–947. http://dx.doi.org/10.1016/j.foodchem.2010.07.031
Sun, B., Yan, H., Zhang, F., et al., 2012. Effects of plant hormones on main health-promoting compounds and antioxidant capacity of Chinese kale. Food Res. Int., 48(2):359–366. http://dx.doi.org/10.1016/j.foodres.2012.04.021
Teng, S., Keurentjes, J., Bentsink, L., et al., 2005. Sucrosespecific induction of anthocyanin biosynthesis in Arabidopsis requires the MYB75/PAP1 gene. Plant Physiol., 139(4):1840–1852. http://dx.doi.org/10.1104/pp.105.066688
Wang, J., Gu, H., Yu, H., et al., 2012. Genotypic variation of glucosinolates in broccoli (Brassica oleracea var. italica) florets from China. Food Chem., 133(3):735–741. http://dx.doi.org/10.1016/j.foodchem.2012.01.085
Wei, J., Miao, H., Wang, Q., 2011. Effect of glucose on glucosinolates, antioxidants and metabolic enzymes in Brassica sprouts. Sci. Hort., 129(4):535–540. http://dx.doi.org/10.1016/j.scienta.2011.04.026
Yuan, G., Sun, B., Yuan, J., et al., 2009. Effects of different cooking methods on health-promoting compounds of broccoli. J. Zhejiang Univ.-Sci. B (Biomed. & Biotechnol.), 10(8):580–588. http://dx.doi.org/10.1631/jzus.B0920051
Yuan, G., Bo, S., Jing, Y., et al., 2010a. Effect of 1-methylcyclopropene on shelf life, visual quality, antioxidant enzymes and health-promoting compounds in broccoli florets. Food Chem., 118(3):774–781. http://dx.doi.org/10.1016/j.foodchem.2009.05.062
Yuan, G., Wang, X., Guo, R., et al., 2010b. Effect of salt stress on phenolic compounds, glucosinolates, myrosinase and antioxidant activity in radish sprouts. Food Chem., 121(4):1014–1019. http://dx.doi.org/10.1016/j.foodchem.2010.01.040
Zang, Y., Ge, J., Huang, L., et al., 2015. Leaf and root glucosinolate profiles of Chinese cabbage (Brassica rapa ssp.pekinensis) as a systemic response to methyl jasmonate and salicylic acid elicitation. J. Zhejiang Univ.-Sci. B (Biomed. & Biotechnol.), 16(8):696–708. http://dx.doi.org/10.1631/jzus.B1400370
Zhang, Y., Zhen, L., Tan, X., et al., 2014. The involvement of hexokinase in the coordinated regulation of glucose and gibberellin on cell wall invertase and sucrose synthesis in grape berry. Mol. Biol. Rep., 41(12):7899–7910. http://dx.doi.org/10.1007/s11033-014-3683-7
Al-Fatlawi, A.A., Abbas, A., Zafaryab, M., et al., 2014. Rhein induced cell death and apoptosis through caspase dependent and associated with modulation of p53, Bcl-2/Bax ratio in human cell lines. Int. J. Pharm. Pharmaceut. Sci., 6(2):515–519.
Andlauer, W., Kolb, J., Stehle, P., et al., 2000. Absorption and metabolism of genistein in isolated rat small intestine. J. Nutr., 130(4):843–846.
Baglia, M.L., Gu, K., Zhang, X., et al., 2015. Soy isoflavone intake and bone mineral density in breast cancer survivors. Cancer Causes Control, 26(4):571–580. http://dx.doi.org/10.1007/s10552-015-0534-3
Banerjee, S., Ali, S., Azmi, A., et al., 2012. Abstract 2698: improved therapeutic activity of isoflavone-G2535 and docetaxel combination in hormone refractory prostate cancer. Cancer Res., 72(8 Suppl.):2698. http://dx.doi.org/10.1158/1538-7445.AM2012-2698
Budhathoki, S., Joshi, A.M., Ohnaka, K., et al., 2011. Soy food and isoflavone intake and colorectal cancer risk: the fukuoka colorectal cancer study. Scand. J. Gastroenterol., 46(2):165–172. http://dx.doi.org/10.3109/00365521.2010.522720
Chen, J., Hou, R., Zhang, X., et al., 2014. Calycosin suppresses breast cancer cell growth via ER?dependent regulation of IGF-1R, p38 MAPK and PI3K/Akt pathways. PLoS ONE, 9(3):e91245. http://dx.doi.org/10.1371/journal.pone.0091245
Choi, E.J., Kim, G.H., 2013. Antiproliferative activity of daidzein and genistein may be related to ERα/c-erbB-2 expression in human breast cancer cells. Mol. Med. Rep., 7(3):781–784. http://dx.doi.org/10.3892/mmr.2013.1283
Cotrim, C.Z., Fabris, V., Doria, M.L., et al., 2013. Estrogen receptor β growth-inhibitory effects are repressed through activation of MAPK and PI3K signalling in mammary epithelial and breast cancer cells. Oncogene, 32(19):2390–2402. http://dx.doi.org/10.1038/onc.2012.261
Cui, M.L., Yang, H.Y., He, G.Q., 2015. Submerged fermentation production and characterization of intracellular triterpenoids from Ganoderma lucidum using HPLCESI-MS. J. Zhejiang Univ.-Sci. B (Biomed. & Biotechnol.), 16(12):998–1010. http://dx.doi.org/10.1631/jzus.B1500147
Ewe, J.A., Wan-Abdullah, W.N., Alias, A.K., et al., 2012. Enhanced growth of lactobacilli and bioconversion of isoflavones in biotin-supplemented soymilk by electroporation. Int. J. Food Sci. Nutr., 63(5):580–596. http://dx.doi.org/10.3109/09637486.2011.641940
Ferlay, J., Soerjomataram, I., Dikshit, R., et al., 2015. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer, 136(5):E359–E386. http://dx.doi.org/10.1002/ijc.29210
Gong, Y., Li, Y., Lu, Y., et al., 2011. Bioactive tanshinones in Salvia miltiorrhiza inhibit the growth of prostate cancer cells in vitro and in mice. Int. J. Cancer, 129(5):1042–1052. http://dx.doi.org/10.1002/ijc.25678
Guo, X.Y., Liu, D., Ye, M., et al., 2013. Structural characterization of minor metabolites and pharmacokinetics of ganoderic acid C2 in rat plasma by HPLC coupled with electrospray ionization tandem mass spectrometry. J. Pharm. Biomed. Anal., 75:64–73. http://dx.doi.org/10.1016/j.jpba.2012.11.024
Handa, C.L., Couto, U.R., Vicensoti, A.H., et al., 2014. Optimisation of soy flour fermentation parameters to produce beta-glucosidase for bioconversion into aglycones. Food Chem., 152:56–65. http://dx.doi.org/10.1016/j.foodchem.2013.11.101
Hati, S., Vij, S., Singh, B.P., et al., 2015. β-Glucosidase activity and bioconversion of isoflavones during fermentation of soymilk. J. Sci. Food Agric., 95(1):216–220. http://dx.doi.org/10.1002/jsfa.6743
Hsin, I.L., Ou, C.C., Wu, M.F., et al., 2015. GMI, an immunomodulatory protein from Ganoderma microsporum, potentiates cisplatin-induced apoptosis via autophagy in lung cancer cells. Mol. Pharm., 12(5):1534–1543. http://dx.doi.org/10.1021/mp500840z
Indran, I.R., Tufo, G., Pervaiz, S., et al., 2011. Recent advances in apoptosis, mitochondria and drug resistance in cancer cells. BBA-Bioenergetics, 1807(6):735–745. http://dx.doi.org/10.1016/j.bbabio.2011.03.010
Kang, D., Mutakin, M., Levita, J., 2015. Computational study of triterpenoids of Ganoderma lucidum with aspartic protease enzymes for discovering HIV-1 and plasmepsin inhibitors. Int. J. Chem., 7(1):62–68. http://dx.doi.org/10.5539/ijc.v7n1p62
Keypour, S., Rafati, H., Riahi, H., et al., 2010. Qualitative analysis of ganoderic acids in Ganoderma lucidum from Iran and China by RP-HPLC and electrospray ionisationmass spectrometry (ESI-MS). Food Chem., 119(4):1704–1708. http://dx.doi.org/10.1016/j.foodchem.2009.09.058
Kim, H.M., Paik, S.Y., Ra, K.S., et al., 2006. Enhanced production of exopolysaccharides by fed-batch culture of Ganoderma resinaceum DG-6556. J. Microbiol., 44(2):233–242.
Kurahashi, N., Iwasaki, M., Sasazuki, S., et al., 2007. Soy product and isoflavone consumption in relation to prostate cancer in Japanese men. Cancer Epidemiol. Biomarkers Prev., 16(3):538–545. http://dx.doi.org/10.1158/1055-9965.EPI-06-0517
Lee, S.Y., Rhee, H.M., 1990. Cardiovascular effects of mycelium extract of Ganoderma lucidum: inhibition of sympathetic outflow as a mechanism of its hypotensive action. Chem. Pharm. Bull., 38(5):1359–1364. http://dx.doi.org/10.1248/cpb.38.1359
Li, Y., Kong, D., Ahmad, A., et al., 2012a. Epigenetic deregulation of miR-29a and miR-1256 by isoflavone contributes to the inhibition of prostate cancer cell growth and invasion. Epigenetics, 7(8):940–949. http://dx.doi.org/10.4161/epi.21236
Li, Y., Kong, D., Ahmad, A., et al., 2012b. Targeting bone remodeling by isoflavone and 3,3’-diindolylmethane in the context of prostate cancer bone metastasis. PLoS ONE, 7(3):e33011. http://dx.doi.org/10.1371/journal.pone.0033011
Lim, J.C.W., Chan, T.K., Ng, D.S., et al., 2012. Andrographolide and its analogues: versatile bioactive molecules for combating inflammation and cancer. Clin. Exp. Pharmacol. Physiol., 39(3):300–310. http://dx.doi.org/10.1111/j.1440-1681.2011.05633.x
Liu, F., Bardhan, K., Yang, D., et al., 2012. NF-κB directly regulates Fas transcription to modulate Fas-mediated apoptosis and tumor suppression. J. Biol. Chem., 287(30):25530–25540. http://dx.doi.org/10.1074/jbc.M112.356279
Liu, J., Zhang, C., Hu, W., et al., 2015. Tumor suppressor p53 and its mutants in cancer metabolism. Cancer Lett., 356(2):197–203. http://dx.doi.org/10.1016/j.canlet.2013.12.025
Loganathan, J., Jiang, J., Smith, A., et al., 2014. The mushroom Ganoderma lucidum suppresses breast-to-lung cancer metastasis through the inhibition of pro-invasive genes. Int. J. Oncol., 44(6):2009–2015. http://dx.doi.org/10.3892/ijo.2014.2375
Maitan-Alfenas, G.P., Lorena, G.A., de Almeida, M.N., et al., 2014. Hydrolysis of soybean isoflavones by Debaryomyces hansenii UFV-1 immobilised cells and free β-glucosidase. Food Chem., 146:429–436. http://dx.doi.org/10.1016/j.foodchem.2013.09.099
Mayola, E., Gallerne, C., Esposti, D.D., et al., 2011. Withaferin a induces apoptosis in human melanoma cells through generation of reactive oxygen species and downregulation of Bcl-2. Apoptosis, 16(10):1014–1027.
Mense, S.M., Hei, T.K., Ganju, R.K., et al., 2008. Phytoestrogens and breast cancer prevention: possible mechanisms of action. Environ. Health Perspect., 116(4):426–433.
Miranda, C.F., Morales-Cruz, M., Suarez, B., et al., 2014. Effect of cytochrome c modification with co-polymer on its apoptotic activity for cancer treatment (LB248). FASEB J., 28(1 Suppl.):248.
Munck, L., Jorgensen, K.G., Ruud-Hansen, J., et al., 1989. The EBC methods for determination of high molecular weight β-glucan in barley, malt, wort and beer. J. Inst. Brewing, 95(2):79–82. http://dx.doi.org/10.1002/j.2050-0416.1989.tb04612.x
Ollberding, N.J., Lim, U., Wilkens, L.R., et al., 2012. Legume, soy, tofu, and isoflavone intake and endometrial cancer risk in postmenopausal women in the multiethnic cohort study. J. Natl. Cancer Inst., 104(1):67–76. http://dx.doi.org/10.1093/jnci/djr475
Prietsch, R.F., Monte, L.G., da Silva, F.A., et al., 2014. Genistein induces apoptosis and autophagy in human breast MCF-7 cells by modulating the expression of proapoptotic factors and oxidative stress enzymes. Mol. Cell Biochem., 390(1-2):235–242. http://dx.doi.org/10.1007/s11010-014-1974-x
Priyadarsini, R.V., Murugan, R.S., Maitreyi, S., et al., 2010. The flavonoid quercetin induces cell cycle arrest and mitochondria-mediated apoptosis in human cervical cancer (HeLa) cells through p53 induction and NF-κB inhibition. Eur. J. Pharmacol., 649(1-3):84–91. http://dx.doi.org/10.1016/j.ejphar.2010.09.020
Selent, J., Kaczor, A.A., Guixa-Gonzalez, R., et al., 2013. Rational design of the survivin/CDK4 complex by combining protein-protein docking and molecular dynamics simulations. J. Mol. Model., 19(4):1507–1514. http://dx.doi.org/10.1007/s00894-012-1705-8
Srivastava, K., Singh, A.K., Khan, K., et al., 2014. Assessment of enhancement of peak bone gain by isoflavone enriched standardized soy extract in female rats. J. Funct. Foods, 7:314–321. http://dx.doi.org/10.1016/j.jff.2014.01.029
Suarez-Jimenez, G.M., Burgos-Hernandez, A., Ezquerra-Brauer, J.M., 2012. Bioactive peptides and depsipeptides with anticancer potential: sources from marine animals. Marine Drugs, 10(12):963–986. http://dx.doi.org/10.3390/md10050963
Szliszka, E., Czuba, Z.P., Sędek, L., et al., 2011. Enhanced TRAIL-mediated apoptosis in prostate cancer cells by the bioactive compounds neobavaisoflavone and psoralidin isolated from Psoralea corylifolia. Pharm. Rep., 63(1):139–148. http://dx.doi.org/10.1016/S1734-1140(11)70408-X
Tang, W., Liu, J.W., Zhao, W.M., et al., 2006. Ganoderic acid T from Ganoderma lucidum mycelia induces mitochondria mediated apoptosis in lung cancer cells. Life Sci., 80(3):205–211. http://dx.doi.org/10.1016/j.lfs.2006.09.001
Thomas, S., Quinn, B.A., Das, S.K., et al., 2013. Targeting the Bcl-2 family for cancer therapy. Exp. Opin. Therapeut. Tar., 17(1):61–75. http://dx.doi.org/10.1517/14728222.2013.733001
Titiek, F., Umar, S., Cahyanto, M., et al., 2013. Effect of indigenous lactic acid bacteria fermentation on enrichment of isoflavone and antioxidant properties of kerandang (Canavalia virosa) extract. Int. Food Res. J., 20(5):2945–2950.
Tse, G., Eslick, G.D., 2016. Soy and isoflavone consumption and risk of gastrointestinal cancer: a systematic review and meta-analysis. Eur. J. Nutr., 55(1):63–73. http://dx.doi.org/10.1007/s00394-014-0824-7
Tsuboy, M.S., Marcarini, J.C., de Souza, A.O., et al., 2014. Genistein at maximal physiologic serum levels induces G0/G1 arrest in MCF-7 and HB4a cells, but not apoptosis. J. Med. Food, 17(2):218–225. http://dx.doi.org/10.1089/jmf.2013.0067
van Meerloo, J., Kaspers, G.J., Cloos, J., 2011. Cell sensitivity assays: the MTT assay. In: Cree, I. (Ed.), Cancer Cell Culture. Methods in Molecular Biology (Methods and Protocols), Vol. 731. Humana Press, p.237–245. http://dx.doi.org/10.1007/978-1-61779-080-5_20
Varfolomeev, E., Goncharov, T., Vucic, D., 2015. Roles of c-IAP proteins in TNF receptor family activation of NF-κB signaling. In: May, M. (Ed.), NF-κB. Methods in Molecular Biology, Vol. 1280. Humana Press, New York, p.269–282. http://dx.doi.org/10.1007/978-1-4939-2422-6_15
Wei, J., Bhatt, S., Chang, L.M., et al., 2012. Isoflavones, genistein and daidzein, regulate mucosal immune response by suppressing dendritic cell function. PLoS ONE, 7(10):e47979. http://dx.doi.org/10.1371/journal.pone.0047979
Xu, C.F., Wu, A.R., Zhu, H., et al., 2013. Melatonin is involved in the apoptosis and necrosis of pancreatic cancer cell line SW-1990 via modulating of Bcl-2/Bax balance. Biomed. Pharmacother., 67(2):133–139. http://dx.doi.org/10.1016/j.biopha.2012.10.005
Yan, L., Spitznagel, E.L., Bosland, M.C., 2010. Soy consumption and colorectal cancer risk in humans: a metaanalysis. Cancer Epidem. Biomar. Prev., 19(1):148–158. http://dx.doi.org/10.1158/1055-9965.EPI-09-0856
Yeo, S.K., Liong, M.T., 2010. Angiotensin I-converting enzyme inhibitory activity and bioconversion of isoflavones by probiotics in soymilk supplemented with prebiotics. Int. J. Food Sci. Nutr., 61(2):161–181. http://dx.doi.org/10.3109/09637480903348122
Yeom, S.J., Kim, B.N., Kim, Y.S., et al., 2012. Hydrolysis of isoflavone glycosides by a thermostable beta-glucosidase from Pyrococcus furiosus. J. Agric. Food Chem., 260(6):1535–1541. http://dx.doi.org/10.1021/jf204432g
Yin, L.J., Tai, H.M., Lee, H.H., et al., 2014. Proteolysis and lactobacillus fermentation effects on the isoflavones transformation and removal of anti-nutritional factors of soy bean. J. Mar. Sci. Technol., 22(4):525–530.
Zhao, W., Xu, J.W., Zhong, J.J., 2011. Enhanced production of ganoderic acids in static liquid culture of Ganoderma lucidum under nitrogen-limiting conditions. Bioresour. Technol., 102(17):8185–8190. http://dx.doi.org/10.1016/j.biortech.2011.06.043
Zhou, C., Lin, H., Ge, X., et al., 2015. The effects of dietary soybean isoflavones on growth, innate immune responses, hepatic antioxidant abilities and disease resistance of juvenile golden pompano Trachinotus ovatus. Fish Shellfish Immunol., 43(1):158–166. http://dx.doi.org/10.1016/j.fsi.2014.12.014
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cui, Ml., Yang, Hy. & He, Gq. Apoptosis induction of colorectal cancer cells HTL-9 in vitro by the transformed products of soybean isoflavones by Ganoderma lucidum . J. Zhejiang Univ. Sci. B 18, 1101–1112 (2017). https://doi.org/10.1631/jzus.B1700189
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1631/jzus.B1700189