Abstract
Elsholtzia splendens (Lamiaceae) is a copper-tolerant plant species growing on copper deposits in the south of China. Chromatographic separation of n-BuOH extracts from the flowering aerial biomass afforded apigenin-7-O-β-d-glycoside, using macroporous resin, Sephadex™ LH-20 gel, polyamide resin as well as preparative high-performance liquid chromatography (P-HPLC) columns. Chemical structure was elucidated using HPLC/ESI-MS (electrospray ionization-mass spectrometry), Fourier transform infrared (FTIR), and 1D- and 2D-nuclear magnetic resonance (NMR). Apigenin-7-O-β-d-glycoside could be the post-harvesting product from E. splendens biomass.
中文概要
目 的
建立从铜耐性植物海州香薷地上部提取分离芹菜素糖苷的工艺技术, 探索铜耐性植物海州香薷资源化途径。
创新点
结合使用大孔树脂、凝胶树脂、聚酰胺树脂多种色谱柱技术, 从铜耐性植物海州香薷中提取分离纯度达98%的黄酮苷类化合物芹菜素糖苷。
方 法
以铜耐性植物海州香薷为原料, 用60%乙醇溶液浸提, 提取液依次使用石油醚、乙酸乙酯、饱和正丁醇萃取。实验中, 对饱和正丁醇萃取层, 使用D101 型大孔吸附树脂粗分离提取, Sephadex™ LH-20 型凝胶筛分分离, 并借助半制备液相和聚酰胺树脂分离纯化, 得到芹菜素糖苷单体(图1)。利用紫外光谱、液相色谱–质谱联用、红外光谱、一维和二维核磁图谱, 鉴定芹菜素糖苷结构(图2∼5)。
结 论
芹菜素糖苷是铜耐性植物海州香薷体内的天然产物, 利用色谱技术可以从海州香薷植物体中分离出芹菜素糖苷单体。
Similar content being viewed by others
1 Introduction
The Chinese native herb Elsholtzia splendens Nakai (Lamiaceae) was first recognized as a copper indicator during copper ore exploration in the 1950s, as it was the dominant plant species colonizing the mining sites (Xie and Xu, 1952). E. splendens has evolved to tolerate and acclimate to high copper toxicity, which affects photosynthesis metabolism (Peng et al., 2013). Interestingly, ingredients from the aerial biomass of E. splendens have been used in North-East Asia in folk and conventional medicine for treating coughs, headaches, and inflammation, and also for anti-oxidative activities (Kim et al., 2003; Choi et al., 2007; 2008; Choi and Kim, 2008a; 2008b; Chung and Kim, 2010). Apigenin (5,7,4′-trihydroxyflavone) and luteolin (5,7,3′,4′-tetrahydroxyflavone) were the flavone ingredients of interest in ethyl acetate extracts of flowering E. splendens biomass, owing to their high inhibitory properties against cancer cell lines (Peng et al., 2014). Flavones are active due to the presence of phenolic hydroxyls and carbonyls. These phenolic hydroxyls are always methoxylated and transformed as their corresponding glycoside, resulting in the production of the flavone glycoside. For instance, apigenin-7-O-β-d-glycoside (cosmosiin) isolated from Lonicera gracilipes var. glandulosa can inhibit the growth of various human cancer cells (Kikuchi and Matsuda, 1996).
A study on flowering E. splendens biomass indicated that the total flavonoids from the n-BuOH extracts exhibited significant concentration-dependent vasorelaxation in endothelium-intact rings of rat, which were not abolished but were significantly reduced by the removal of endothelium (Wang et al., 2014). Therefore, we focused on the n-BuOH extracts of the flowering aerial biomass of E. splendens, using macroporous resin (MR), Sephadex™ LH-20, polyamide (PA) resin, and preparative high-performance liquid chromatography (P-HPLC) columns during chromatographic column (CC) separation. This led to the isolation of apigenin-7-O-β-d-glycoside, and we present its isolation and structural elucidation here.
2 Materials and methods
2.1 General
Column chromatography was performed on Macroporous resin D101 (Shanghai Mosu Chemicals Co., Ltd., China), Sephadex™ LH-20 (25–100 µm, Pharmacia Fine Chemical Co., Ltd., Sweden), and PA resin (100-200 mesh, Qingdao Haiyang Chemical Co., China). Thin layer chromatography (TLC) was carried out on PA pre-coated plates (Taizhou Chemical Co., China) with MeOH-H2O (3:1, v/v, the same below) and/or EtOAc-MeOH (5:1) as developing agents. The spots were detected by spraying with 3% (0.03 g/ml) FeCl3-ethanol solution. Ultraviolet (UV) spectra were obtained on a UV 210A spectrometer (Shimadzu, Kyoto, Japan). Structural elucidations were achieved using standard spectroscopic techniques, such as Fourier transform infrared (FTIR), 1H nuclear magnetic resonance (NMR)/13C NMR, and HPLC/ESI-MS (electrospray ionization-mass spectrometry). FTIR spectra were recorded on a Nicolet Nexus-670 spectrometer with a Ge/KBr beam splitter and a deuterated triglycine sulfate (DTGS) detector at room temperature. The wavenumber accuracy was 0.01 cm−1; 40 scans were recorded at a resolution of 1 cm−1 and averaged for the spectra. 1H and 13C NMR spectra were recorded with a Bruker AVIII 500 spectrometer (500 MHz for 1H and 125 MHz for 13C), using tetra-methylsilane (TMS) as an internal standard (δ=0). The values of chemical shifts were given in δ (ppm, 10−6) and coupling constants in J (Hz); 1H spectra were referenced to the residual dimethyl sulfoxide (DMSO) peak at δ 2.5 and 13C NMR at δ 39.5. Prior to analysis, samples were dissolved in DMSO-d6 during the transfer to the NMR tube. Nuclear Overhauser effect (NOE) NMR was recorded with DRX-500 spectrometers (400 MHz for 1H). ESI-MS spectra were recorded on an Agilent 6460 Triple Quad liquid chromatography (LC)/ESI-MS (VG, Manchester, UK) in both negative and positive modes. Further, purity of compound was assessed by an Agilent 6460 Triple Quad LC/ESI-MS, which was equipped with a Zorbax SB C18 column (250 mm× 4.6 mm; 5 µm particle size); samples were detected at 230 nm in a G1314E 1290 VWD UV detector with acetonitrile/H2O (60%−0%, v/v) as mobile phase at a flow rate of 1 ml/min. Each fraction during separation was sampled and analyzed by HPLC on an Agilent Technology 1200 series, equipped with an eclipse XDB C18 column (250 mm×<4.6 mm, 5 µm particle size) in a G1316C 1290 TCC thermostat-monitored column compartment and G1314B VWD UV detector (samples detected at 230 nm).
2.2 Plant materials and extraction
The flowering aerial biomass of adult E. splendens plants was harvested from a copper-rich site located at Fuyang County, Zhejiang Province, East China, in October 2014. After being naturally air-dried, the leaves and flowers of plants were ground into powder. The powdered E. splendens (2 kg) biomass was extracted with 60% ethanol (3×30 L) at room temperature for one month. After filtration, the filtrate was vacuum-concentrated to yield a crude extract (520 g), which was successively partitioned between H2O (3×3000 ml) and petroleum ether (3×3000 ml), EtOAc (3×3000 ml) and H2O-saturated n-BuOH (3×3000 ml), yielding deep-brown n-BuOH extract (144 g).
2.3 Isolation of the compound with MR and Sephadex™ columns
The n-BuOH extract (deep-brown) was chromatographed on an MR column of D101 type, and eluted with a gradient H2O-EtOH from 90:10 to 5:95, (v/v); each proportion used 4 to 8 L, and afforded yellow-brown extract (20.2 g) from the 70% (v/v, the same below) ethanol-eluted fractionation. Yellowbrown extract was dissolved in 70% MeOH-H2O, then chromatographed over a Sephadex™ LH-20 gel column (eluted with 70% and 95% MeOH-H2O), giving Fraction 1 (0.58 g, Pellet A).
2.4 Purification of the compound with P-HPLC and PA
Pellet A (0.58 g) was isolated on an Agilent P-HPLC, which was equipped with a Sepax amethyst C18-H column (21.2 mm×250 mm, 5 µm particle size), with 40% MeOH-H2O as mobile phase. The partitions that peaked at around 10–15 min were collected (230 nm) and vacuum-concentrated, giving Subfraction 1.1 (0.10 g, Pellet A1) with a purity of 80%. Pellet A1 was subjected to a PA column, and eluted with EtOAc/MeOH (5:1, v/v), affording a compound (yellow powder) from the partitions through the PA column (TLC, R f =0.6, 254 nm) with a purity of 98%.
3 Results and discussion
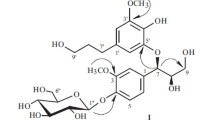
Column chromatography (MR, Sephadex™ LH-20, P-HPLC, and PA) was performed on the n-BuOH extracts of flowering E. splendens biomass, affording a compound (Fig. 1) with a purity of 98%, as determined by HPLC analyses (Fig. 2). The chemical structures were elucidated by HPLC/ESI-MS, FTIR, and NMR (Figs. 2–4). The compound was a light-yellow amorphous powder; its molecular formula was determined to be C21H20O10 as derived from its positive ESI-MS (m/z 433.2 [M-H]+, 887.3 [2M-Na]+) and negative ESI-MS (m/z 431.1 [M-H]−, 863.3 [2M-H]−) (Fig. 2), and further confirmed by a 13C NMR spectrum which has 19 efficient carbon signals (Fig. 4), indicating the presence of 12 degrees of unsaturation. The peak at m/z 271.1 [M-H]+ from positive ESI-MS exhibited the presence of a flavone skeleton (She et al., 2009). The UV maxima (λmax) (MeOH) were at 267 nm (Band II) and 337 nm (Band I), characteristics of a flavone skeleton (Malikov and Yuldashev, 2002).
An FTIR spectrum (KBr) (Fig. 3) exhibited the occurrence of hydroxyl (3423 cm−1), a weak peak attributed to a stretching vibration of aromatic C-H (3139 cm−1), saturated hydrocarbon (2925 cm−1), γ-pyrone carbonyl (1655 cm−1), aromatic C=C (1609, 1590, 1510 cm−1), aromatic hydroxyl (1371, 1276 cm−1), aromatic C-H (1499, 1452 cm−1), aromatic rings (1416, 910, 834, 771 cm−1), and glycoside C-O (1100, 1082, 1030 cm−1) (Berashvili et al., 2005). An IR spectrum (KBr) at 3423, 1655, and 1590 cm−1 had the characteristic bands of apigenin.
1H NMR spectra (Table 1 and Fig. 4a) further confirmed the presence of apigenin and glucose moieties in the structure. The occurrence of an apigenin skeleton was viewed from a hydroxyl δH at 12.97 (s, OH-5), two doublets δH at 6.43 (d, J=2.2 Hz, H-6) and δH at 6.82 (d, J=2.2 Hz, H-8) on the A-ring; A2B2-type aromatic δH at 7.95 (d, J=8.9 Hz, H-2′, H-6′) and δH at 6.93 (d, J=8.9 Hz, H-3′, H-5′), as well as a hydroxyl δH at 10.51 (s, OH-4′) on the B-ring; together with an olefinic δH at 6.87 (s, H-3) on a flavone C-ring. 1H NMR data fit exactly with previously reported data in the literature (Shen et al., 1993; Berashvili et al., 2005). Besides this, glycosidic δH at 5.44 (d, J=7.4 Hz, H-1″) and δC at 99.9 (C-1″) were evident in the 1H and 13C NMR spectra. The multiplet δH at 3.27–3.47 (5H, m, H-3″–H-6″) was assignable to the coupling between protons and methylene protons of the glucosyl ring. Proton δH at 3.71 (m, H-2″), hydroxyl δH at 5.12 (s, OH-2″), δH at 5.07 (s, OH-3″), δH at 5.05 (s, OH-4″), and δH at 4.65 (s, OH-6″) were assigned in the glycosidic ring.
Analyses of the 13C NMR spectrum (Table 1 and Fig. 4b) revealed the existence of 21 carbons, including a hexose moiety at δC (99.9, 73.5, 77.6, 69.9, 76.9, and 63.5). The 13C NMR spectrum also exhibited the presence of δC (157.4, 100.3, 164.7, and 95.3) for the A-ring, δC (163.4, 103.5, 182.5, 161.9, and 105.8) for the C-ring, and δC (121.4, 129.1, 116.5, and 161.6) for the B-ring of the flavone. The NOESY NMR spectra suggested an NOE association between the anomeric proton (H-1″) of the glucoside moiety and the H-8 singlet on the A-ring of the aglycone, confirming the attachment of the sugar unit to the 7-OH functional group (Fig. 5a). Also, the glycosidic linkage at C-7 of the aglycon was viewed from the upfield shift of δC (C-7) as compared with apigenin, which can be further deduced from the existence of δC at 99.9 (C-1″) (Table 1 and Fig. 4b). The large H-1″ coupling constant of 6.8 Hz between H-1″ and H-2″ indicated a β-coupled glucose. The glycoside stereochemistry was affirmed by positive NOEs between H-1″, -3″, and -5″ (Fig. 5b) and by the characteristic 1H chemical shifts (Fig. 4a). Therefore, the structure of the purified compound was elucidated as apigenin-7-O-β-d-glycoside, in accordance with the reported data in the literature (Yang et al., 2007; Liu et al., 2012).
4 Conclusions
Apigenin-7-O-β-d-glycoside could be the post-harvesting product from E. splendens biomass.
Chromatographic column separation was set up for the preparation and purification of apigenin-7-O-β-d-glycoside in the n-BuOH extracts from the flowering aerial biomass of E. splendens.
Compliance with ethics guidelines
Hong-yun PENG, Xue-hong ZHANG, and Jin-zhong XU declare that they have no conflict of interest.
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Berashvili, D.T., Alaniya, M.D., Bakuridze, A.D., et al., 2005. Apigenin glucuronide from Perilla nankinensis leaves. Chem. Nat. Compd., 41(1):97–98. http://dx.doi.org/10.1007/s10600-005-0086-y
Choi, E.J., Kim, G.H., 2008a. In vivo antioxidative characteristics of extracts from the aromatic herb Elsholtzia splendens. Food Sci. Biotechnol., 17(5):1128–1130.
Choi, E.J., Kim, G.H., 2008b. Effect of Elsholtzia splendens extracts on the blood lipid profile and hepatotoxicity of the mice. Food Sci. Biotechnol., 17:413–416.
Choi, E.J., Lee, Y.S., Kim, G.H., 2007. Antioxidative characteristics of extracts from aromatic herb Elsholtzia splendens. Food Sci. Biotechnol., 16:489–492.
Choi, E.J., Kim, T., Kim, G.H., 2008. Antioxidant effects of Elsholtzia splendens extract on DMBA-induced oxidative stress in mice. Food Sci. Biotechnol., 17:1341–1344.
Chung, M.S., Kim, G.H., 2010. Effects of Elsholtzia splendens and Cirsium japonicum on premenstrual syndrome. Nutr. Res. Pract., 4(4):290–294. http://dx.doi.org/10.4162/nrp.2010.4.4.290
Kikuchi, M., Matsuda, N., 1996. Flavone glycosides from Lonicera gracilipes var. glandulosa. J. Nat. Prod., 59(3):314–315. http://dx.doi.org/10.1021/np960180j
Kim, D.W., Son, K.H., Chang, H.W., et al., 2003. Antiinflammatory activity of Elsholtzia splendens. Arch. Pharm. Res., 26:232–236. http://dx.doi.org/10.1007/BF02976835
Liu, J., Chen, L., Cai, S., et al., 2012. Semisynthesis of apigenin and acacetin-7-O-β-d-glycosides from naringin and their cytotoxic activities. Carbohyd. Res., 357:41–46. http://dx.doi.org/10.1016/j.Carres.2012.05.013
Malikov, V.M., Yuldashev, M.P., 2002. Phenolic compounds of plants of the Scutellaria L. genus. Distribution, structure, and properties. Chem. Nat. Compd., 38(4):358–406. http://dx.doi.org/10.1023/A:1021638411150
Peng, H.Y., Kroneck, P.M.H., Kupper, H., 2013. Toxicity and deficiency of copper in Elsholtzia splendens affect photosynthesis biophysics, pigments and metal accumulation. Environ. Sci. Technol., 47(12):6120–6128. http://dx.doi.org/10.1021/es3050746
Peng, H.Y., Xing, Y., Gao, L.L., et al., 2014. Simultaneous separation of apigenin, luteolin and rosmarinic acid from the aerial parts of the copper-tolerant plant Elsholtzia splendens. Environ. Sci. Pollut. Res., 21(13):8124–8132. http://dx.doi.org/10.1007/s11356-014-2747-5
She, G.M., Guo, Z.Q., Lv, H.N., et al., 2009. New flavonoid glycosides from Elsholtzia rugulosa Hemsl. Molecules, 14(10):4190–4196. http://dx.doi.org/10.3390/molecules14104190
Shen, C.C., Chang, Y.S., Ho, L.K., 1993. Nuclear magnetic resonance studies of 5,7-dihydroxy flavonoids. Phytochemistry, 34(3):843–845. http://dx.doi.org/10.1016/0031-9422(93)85370-7
Wang, H.P., Lu, J.F., Zhang, G.L., et al., 2014. Endotheliumdependent and-independent vasorelaxant actions and mechanisms induced by total flavonoids of Elsholtzia splendens in rat aortas. Environ. Toxicol. Phar., 38(2): 453–459. http://dx.doi.org/10.1016/j.etap.2014.07.019
Xie, X.J., Xu, Z.B., 1952. Elsholtzia haichouensis—an indicator of copper mine. Geol. Acta, 32:360–368 (in Chinese).
Yang, A.M., Lu, R.H., Shi, Y.P., 2007. Study on flavonoids from Lagotis ramalana Batalin. Chin. Pharm. J., 19:1459–1461 (in Chinese).
Author information
Authors and Affiliations
Corresponding author
Additional information
Project supported by the National Natural Science Foundation of China (No. 21277121)
ORCID: Hong-yun PENG, http://orcid.org/0000-0001-5520-7074
Rights and permissions
About this article
Cite this article
Peng, Hy., Zhang, Xh. & Xu, Jz. Apigenin-7-O-β-d-glycoside isolation from the highly copper-tolerant plant Elsholtzia splendens. J. Zhejiang Univ. Sci. B 17, 447–454 (2016). https://doi.org/10.1631/jzus.B1500242
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1631/jzus.B1500242
Key words
- Chromatographic separation
- Copper-tolerant plant
- Elsholtzia splendens
- Flavonoid glycoside
- Structure elucidation