Abstract
Insect resistance and glyphosate tolerance have been two of the most important traits in the genetic improvement of various crops. In this study, two Bacillus thuringiensis (Bt) insecticidal genes, Cry1Ac and Cry1Ig, and a modified glyphosate-tolerant 5-enolpyruvylshikimate-3-phosphate synthase (EPSPS) gene (G10) were combined into a single transferred DNA (T-DNA) fragment and introduced into rice by Agrobacterium-mediated transformation. A transgenic line with single-copy T-DNA insertion named GAI-14 was found to be highly resistant to striped stem borer and rice leaf roller, and tolerant to glyphosate. Analysis of T-DNA border sequence suggested that the transgenes were inserted at the chromosome 3 and appeared to have not interrupted any known or putative genes. A field trial observed no significant difference in the basic agronomic traits between GAI-14 and the recipient rice.
中文概要
目 的
获得具有高水平表达Cry1Ac、Cry1Ig和EPSPS基因并且具有抗虫、抗草甘膦活性的转基因水稻株。
创新点
Cry1Ig为新发现的苏云金杆菌(Bt)杀虫基因; Cry1Ig与Cry1Ac共同使用有利于延缓害虫产生对Bt杀虫蛋白抗性。
方 法
将Cry1Ac、Cry1Ig和EPSPS(G10)基因的表达框依次插入到同一转移DNA(T-DNA)中, 再将此T-DNA 利用农杆菌介导法转化到水稻中。从转基因后代中, 利用蛋白质印迹法(Western blot)、DNA 印迹法(Southern blot)和热不对称交错聚合酶链式反应(TAIL-PCR)技术, 筛选出能够高水平表达上述三个基因以及T-DNA 单拷贝插入并且未发生明显插入突变的转化株。对于筛选到的转化株, 利用Western blot 进一步分析Cry1Ac、Cry1Ig和G10基因的表达水平; 使用酶联免疫吸附测定(ELISA)检测Cry1Ac 的表达量; 使用棉铃虫、二化螟和稻纵卷叶螟为对象, 测定转基因水稻的抗虫活性; 使用草甘膦喷施法, 测定转基因水稻的抗草甘膦活性。最后, 在大田试验中, 考察转基因水稻与非转基因水稻的基本农艺性状, 观察T-DNA 的插入是否对转基因水稻的生长产生明显的影响。
结 论
本实验最终获得具有高水平表达Cry1Ac、Cry1Ig和G10 基因以及T-DNA 单拷贝插入并且未引起明显变化的转化株GAI-14。GAI-14 对棉铃虫、二化螟、稻纵卷叶螟以及草甘膦均具有明显的抗性。田间试验表明GAI-14 在基本农艺性状上与非转基因水稻无明显差异。
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Herbicide tolerance and insect resistance are two of the most important traits for the genetic improvement of various crops (James, 2013). Herbicide tolerance, mainly glyphosate tolerance, facilitates weed management in a crop field, whilst insect resistance promotes crop production by reducing the damage caused by pests. Currently commercial transgenic crops are usually stacked with insect-resistant and herbicide-tolerant genes as both traits are highly valuable in the production of major crops such as corn and cotton (James, 2013). Multiple insect-resistant genes and herbicide-tolerant genes were stacked in newly developed commercial transgenic crops. For example, a total of eight genes for insect resistance or herbicide tolerance were stacked in the Genuity® SmartStax™ corn released by Monsanto (USA).
Currently the most widely used method for multiple gene stacking is crossing hybridization between different transgenic plants harboring one or two foreign genes. Crossing strategy includes multiple transformations, characterization of each transgenic population, and cross hybridization between different transgenic plants, and thus it is slow and labor-intensive (Halpin, 2005). The introduction of one single transferred DNA (T-DNA) containing multiple gene cassettes is faster and more cost-effective. Using this method, different genes were introduced into a plant and inserted at the same site of the genome by only one round of transformation. The main limitations for linking multiple genes into one T-DNA are the T-DNA size limitation and the uneven gene expression among different genes (Halpin, 2005).
Resistance to Bacillus thuringiensis (Bt) toxin has been reported in several major field insects, including Plutella xylostella (Ferré et al., 1991), Trichoplusia ni (Wang et al., 2006), Spodoptera frugiperda (Storer et al., 2010), Busseola fusca (Kruger et al., 2011), Pectinophora gossypiella (Dhurua and Gujar, 2011), Diabrotica virgifera (Gassmann et al., 2011), and Helicoverpa armigera (Alvi et al., 2012; Zhang et al., 2012). Bt resistance reduces the efficacy of Bt crops and threatens the long-term utilization of Bt insecticidal genes in the transgenic crops. Bt gene stacking strategy introduces different insecticidal genes into plant, and was proved to be an effective way of delaying the development of insect resistance to Bt toxin (Zhao et al., 2003). Thus, Bt genes with different modes of action were usually stacked together in the newly developed transgenic crops.
In this study, two Bt insecticidal genes, Cry1Ac and Cry1Ig, and a modified glyphosate-tolerant 5-enolpyruvylshikimate-3-phosphate synthase (EPSPS) gene (G10) were constructed into a single T-DNA vector and introduced into rice by Agrobacterium-mediated transformation. The Cry1Ac and Cry1Ig were stacked to facilitate a delay in the development of Bt resistance and to enhance the insecticidal activity of the transgenic rice, whilst the G10 was used to confer glyphosate-resistance and also served as the selection marker for transformation. We obtained a single copy insertion transgenic line GAI-14 for use in this study, which is highly resistant to major rice lepidopteran pests and also tolerant to glyphosate. Field trials observed no significant difference in agronomic traits between GAI-14 and its recipient line. The integration of a single T-DNA containing three genes significantly simplifies the gene stacking procedure and will speed the breeding process.
Materials and methods
Rice varieties
Elite rice (Oryza sativa spp. japonica) cultivar Xiushui 134 was used as the recipient for Agrobacterium-mediated transformation. Xiushui 134 is a leading commercial cultivar in northern Zhejiang Province and was originally developed by the Jiaxing Academy of Agricultural Science in Zhejiang Province, China.
Construction of a binary vector for rice transformation
The Cry1Ac, Cry1Ig, and G10 genes were synthesized in accordance with the amino acid sequences of Cry1Ac (GenBank: ADO64599.1), Cry1Ig (GenBank: AGU13866.1), and EPSPS (Swiss-Prot: Q9RVD3.2), respectively, with the codon optimized for expression in rice. The coding sequence for transit peptide of maize acetohydroxy acid synthase (AHAS, GenBank: NP_001151166.1) was linked to the three genes at the 5′ terminus.
The construction of the binary vector was based on pCambia 1300 (Cambia). The G10 expression cassette under the control of maize ubiquitin promoter (pUBi) was inserted into the 1300 plasmid pre-digested with KpnI and XhoI to substitute the hygromycin-resistant gene to generate vector 1300-G10. Subsequently the Cry1Ig expression cassette under the control of pUbi was inserted into the 1300-G10 plasmid pre-digested with HindIII and KpnI to generate 1300-Cry1Ig-G10. Finally the Cry1Ac expression cassette under the control of modified cauliflower 35S promoter (original 35S promoter linked with the first intron of rice Actin1 gene (Zhao et al., 2014)) was inserted into the 1300-Cry1Ig-G10 plasmid pre-digested with HindIII to generate the final vector pCambia1300-Cry1Ac-Cry1Ig-G10 (Fig. 1).
Schematic diagram of the T-DNA vector used for rice transformation
The T-DNA contained three expression cassettes: the cassette for Bt insecticidal gene Cry1Ac under control of modified cauliflower mosaic virus 35S promoter (the original 35S promoter linked with the first intron of rice Actin-1 gene, p35sM), the cassette for Bt insecticidal Cry1Ig under control of maize ubiquitin promoter (pUbi), and the cassette for glyphosate-tolerant 5-enolpyruvylshikimate-3-phosphate synthase (EPSPS) gene (G10) under control of pUbi promoter. Cry1Ac, Cry1Ig, and G10 were synthesized genes fused with the coding sequence for transit peptide derived from maize acetohydroxy acid synthase at their 5′ terminus. RB and LB indicate the right border and left border of the T-DNA, respectively
Agrobacterium-mediated transformation of rice
The T-DNA plasmid was transformed into Agrobacterium tumefaciens LBA4404 by electroporation. Agrobacterium-mediated rice transformation was carried out according to Hiei et al. (1994), with the exception that the Agrobacterium-inoculated calli were selected on the basis of using a culture medium containing 2 mmol/L glyphosate (Sigma-Aldrich, St. Louis, USA).
Western blot analysis
The Western blot analysis was performed as previously described (Zhao et al., 2014). Soluble protein extract was separated on 8% (w/v) polyacrylamide gel and transferred to a polyvinylidene fluoride (PVDF) membrane. After being incubated with a primary antibody, the membrane was then incubated with horseradish peroxidase (HRP)-conjugated secondary antibody (Promega, Wisconsin, USA). Finally the signal was visualized with diaminobenzidine (DAB) substrate. Each sample was detected with primary antibody against Cry1Ac, Cry1Ig, and G10, respectively. The Cry1Ac, Cry1Ig, and G10 proteins, previously expressed by Escherichia coli, were used as a positive control. A protein sample prepared from non-transgenic recipient rice was used as a negative control.
Southern blot analysis
The Southern blot was carried out in accordance with the protocol described in Molecular Cloning (Sambrock and Russel, 2001). Leaf tissue of 0.5 g was used for genome extraction using cetyltrimethylammonium bromide (CTAB) method. Genomic DNA of 100 µg was digested with BamHI. The digested genomic DNA was size-fractionated on 0.7% (w/v) agarose gel and transferred onto a positively charged nylon membrane. The DIG-labelled probe, specific to G10, was prepared as previously described (Li et al., 2013). The signal was detected in accordance with the DIG system manual (Roche, Basel, Switzerland).
Detection of the T-DNA insertion site with thermal asymmetric interlaced-polymerase chain reaction (TAIL-PCR)
TAIL-PCR was carried out as specified by Liu et al. (1995). Primers used were listed in Table 1. Three runs of PCR reaction were carried out: the primary PCR with LB-SPI and AD primers, the secondary PCR with LB-SPII and AD primers, and the tertiary PCR with LB-SPIII and AD primers. PCR product was recovered and sequenced. In accordance with the sequence of the TAIL-PCR product, primers of RBD14-500 and RB-SP (Table 1) were designed to amplify the joint sequence of the right border.
Protein quantification of Cry1Ac
The concentration of Cry1Ac in the leaves of transgenic rice at the tillering stage was determined by enzyme-linked immunosorbent assay (ELISA) as previously described (Zhao et al., 2014). The Enviologix kit AP003 and Cry1Ac standard protein bought from Envirologix (Portland, USA) were used according to the manufacturer’s instructions. The assay was repeated twice. The sample prepared from non-transgenic rice was used to eliminate the basal absorption at 450 nm.
Insect bioassay and glyphosate resistance assay
Insect bioassay was conducted with cotton bollworm (Helicoverpa armigera), striped stem borer (Chilo suppressalis), and rice leaf roller (Cnaphalocrocis medinalis) as previously described by Zhao et al. (2014). Detached leaf bioassay was carried out for striped stem borer. Leaf blades were collected and infested with ten newly hatched neonates. Pre-moistened filter paper was used to keep the leaf blade from drying. The bioassay was conducted in the dark at 28 °C. The result was collected 3 d later. Bioassay for cotton bollworm was carried out by the same method with the exception that each sample of leaf blades was infested with one neonate to avoid cannibalism between the neonates. A total of ten cotton bollworm neonates were used for each rice line. Whole rice plants at the tillering stage grown in the greenhouse were used for the bioassay of rice leaf roller. Each plant was infested with ten newly hatched neonates. Two weeks later, the result was collected. The bioassay was repeated twice for the three different kinds of insects used. Insect mortalities were analyzed using a Kruskal Wallis test in SPSS (IBM, New York, USA).
Glyphosate spraying was carried out as descripted by Zhang et al. (2013). Roundup (Monsanto, La Conner, USA) diluted at 1:140 (v/v) was sprayed onto rice at a 10-cm height at the rate of 0.25 ml/m2. Ten days later the results were recorded. Non-transgenic recipient rice at the same growing stage was used as the control.
Evaluation of agronomic traits
Both the transgenic and non-transgenic recipient rice plants were planted in the field at the Zhejiang University Farm in Changxing, China. The rice field of 667 m2 was divided into six plots. The transgenic rice and control rice were each randomly planted for three plots. At harvest, 30 plants were randomly selected from each plot, and the plant height, number of panicles per plant, and number of grains per panicle were measured. For each plot, rice seeds were collected and dried to measure the 1000-grain weight. The difference between the transgenic and control rice was analyzed by Student’s t-test in SPSS.
Results
Rice transformation and selection of transgenic events
The transgenic T0 plants were first sprayed with glyphosate to deselect any events with no tolerance to, or weak tolerance to, glyphosate. A total of about 100 independent glyphosate-tolerant events were obtained and all were analyzed by Western blot using antiserum against Cry1Ac and Cry1Ig, respectively. A total of 14 transgenic lines were selected for their relatively high expressions of both Cry1Ac and Cry1Ig. The T0 plants of these 14 lines were planted in the rice field and their basic agronomic traits, such as the tiller number, plant height, filled seeds per panicle, and mature date, were evaluated. Events GAI-10, 14, 56, and 71 showed no significant difference in their agronomic traits compared with non-transgenic control plants, and thus were selected for further characterization.
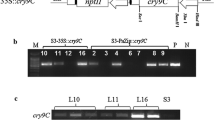
To determine the copy number of the T-DNA inserted, Southern blot analysis was performed for the transgenic lines GAI-10, 14, 56, and 71 using the probe specific to G10 gene. We found that GAI-14 and GAI-71 were single-copy inserted events (Fig. 2).
Determination of the insertion site of T-DNA
To determine the insertion site of the T-DNA at the rice genome, a TAIL-PCR was performed to obtain the genomic sequence that borders the T-DNA of the event GAI-14. By searching the rice genome database, the insertion site of the left border was located at the site of 21 284 675 bp on chromosome 3 (GenBank accession No. AC128645.5) while the insertion site of the right border was located at the site of 21 284 631 bp. Analysis of the sequence at the insertion site suggested a deletion of a 44-base fragment in the rice genomic sequence due to the T-DNA insertion. Bioinformatic analysis indicated that no annotated or putative genes were located at or close to the insertion site, suggesting that the impact of the T-DNA insertion on rice gene expression would likely be minimal.
A similar study found that the insertion site of GAI-71 was located in the second exon of gene LOC_Os12g4220, a predicted K+/H+-antiporter gene, at chromosome 12 and caused a null mutation in this gene. Although we did not observe any abnormal phenotypes in GAI-71, we discarded this line because of its interruption of a possible functional gene.
The homozygous GAI-14 plants were identified at the T1 generation by PCR based on the border genomic sequence of T-DNA insertion. Primers of HoD-F and RBD14-500 (Table 1), which were paired with the rice genomic sequence flanking the T-DNA insertion site, were used to amplify the genomic fragment containing the T-DNA insert site. The plants that failed to generate a PCR product due to the over 10 kb T-DNA insertion were homozygous plants, whilst the plants that generated a product of an expected size of 500 bp were the heterozygous plants. The selected homozygous GAI-14 plants were further characterized and analyzed.
Analysis of the expression of the transgenes in GAI-14
The expression of the transgenes Cry1Ac, Cry1Ig, and G10 was analyzed using a Western blot analysis for the homozygous GAI-14 plants. The results showed that Cry1Ac, Cry1Ig, and G10 were highly expressed in the GAI-14 rice plants (Fig. 3). The sizes of the major Cry1Ac, Cry1Ig, and G10 proteins were equal to those of the control proteins expressed in E. coli, suggesting that the chloroplast signal peptide attached to the N-terminus of these proteins had been correctly processed. We further measured the Cry1Ac expression level using an ELISA kit (Envirologix), and it was estimated at 11 µg/g leaf fresh weight.
Western blot analysis of transgene expression
Six plants were selected. Each sample was blotted with a primary antibody against Cry1Ac (a), Cry1Ig (b), and G10 (c), respectively. Prokaryotic expressed Cry1Ac, Cry1Ig, and G10 were used as a positive control (CK+), respectively. The sample prepared from non-transgenic rice was used as a negative control (CK−)
Insect-resistant and glyphosate-resistant activities of GAI-14
To determine the insecticidal activity of the homozygous GAI-14 plants, neonates of cotton bollworm, striped stem borer, and rice leaf roller were used for the bioassay. Mortalities of all insects feeding on GAI-14 plants were 100% while those on the non-transgenic rice were much lower (Fig. 4). The leaves from GAI-14 were only slightly bitten by cotton bollworm and striped stem borer while non-transgenic rice had suffered significant damage from aforementioned insects (Figs. 5a and 5b). In terms of the rice leaf roller assay, not a single rolled leaf was observed in the GAI-14 plants while several leaves were rolled in the non-transgenic plants (Fig. 5c). These results demonstrate that GAI-14 was highly insect-resistant.
Mortalities of cotton bollworm (CB), striped stem borer (SSB), and rice leaf roller (RLR) feeding on GAI-14
Non-transgenic rice at the same growing stage was used as the control (CK). Data are expressed as mean±standard deviation (n=3). Mortalities of all insects feeding on GAI-14 plants were 100%. ** on the bars indicated extremely significant difference between CK and GAI-14 (P<0.01, Kruskal Wallis test)
In order to determine the glyphosate-tolerant activity of GAI-14, transgenic rice plants growing in a greenhouse were sprayed with Roundup diluted at 1:140 (v/v), the recommended dose for corn filed (glyphosate is not usually used in rice fields). No obvious damage was observed on transgenic plants after they had been sprayed with glyphosate whilst the non-transgenic rice plants were killed (Fig. 6). The results have demonstrated that GAI-14 was highly glyphosate-tolerant.
Analysis of agronomic traits of GAI-14
In order to investigate if there is any alternation of agronomic traits in homozygous GAI-14 compared to the non-transgenic recipient Xiushui 134, a field trial was carried out at our university farm. During harvest-time, the plant height, panicles per plant, grains per panicle, and 1000-grain weight were compared between the GAI-14 and Xiushui 134 (Table 2). The results showed that no obvious difference was observed between the GAI-14 and Xiushui 134 in the aforementioned agronomic traits, suggesting that the introduction of the T-DNA and the expression of the genes it carries did not significantly alter the transgenic rice agronomically.
Discussion
Transgenic crops with more than one trait offer broader agronomic enhancements to meet the needs under complex farming conditions. However, multiple gene stacking in transgenic plants is still problematic in terms of creating a transgenic crop with various desired traits (Halpin, 2005). At present, approved genetically modified crops with multiple genes mainly originate from serial crossing of plants containing single effect gene. Compared with gene stacking by cross hybridization, the introduction of a T-DNA containing multiple genes is a much easier and more effective way to introduce multiple genes (Halpin, 2005). Due to the integration of different genes at the same site, introduction of a multiple-gene T-DNA will greatly speed the breeding process. Some approved genetically modified crops with multiple genes, such as the New Leaf-Plus potato and the InVigor canola, were developed using this method. A crucial limitation in utilizing this approach is the possible uneven expression between different genes. Fortunately, all the three genes are highly expressed in the selected transgenic event GAI-14 in this study.
The development of insect resistance to Bt is a major concern for Bt transgenic crops. The Bt gene stacking strategy is an effective way to cope with the insect resistance to Bt. For instance, transgenic broccoli containing both Cry1Ac and Cry1C Bt genes effectively controlled diamondback moths resistant to either single protein (Cao et al., 2002). Cry1Ig is a newly discovered gene which showed high insecticidal activity to lepidopterans (data unpublished), while Cry1Ac is an elite insecticidal Bt gene which was highly insecticidal to striped stem borer and rice leaf roller (Yang et al., 2012), two major rice pests in China. Furthermore, Ruiz de Escudero et al. (2006) demonstrated that Cry1I shared different binding sites in midgut membrane vesicles from Cry1Ac, suggesting that cross resistance between Cry1Ac and Cry1Ig was unlikely to occur. Therefore, the stacking of Cry1Ac and Cry1Ig will not only enhance protection against insect attack but also delay the development of resistance to Bt toxins.
The transgenic line GAI-14 showed excellent insect-resistance to major pests in rice and high glyphosate-tolerant activity. Although no obvious difference has thus far been observed in the basic agronomic performance between GAI-14 and its recipient non-transgenic rice, further field trials at different locations and over a number of years must be undertaken in order to examine the inheritance stability of the introduced genes and determine the impact of T-DNA insertion on agronomic traits under crop field conditions.
Compliance with ethics guidelines
Qi-chao ZHAO, Ming-hong LIU, Xian-wen ZHANG, Chao-yang LIN, Qing ZHANG, and Zhi-cheng SHEN declare that they have no conflict of interest.
All institutional and national guidelines for the care and use of laboratory animals were followed.
References
Alvi, A.H., Sayyed, A.H., Naeem, M., et al., 2012. Field evolved resistance in Helicoverpa armigera (Lepidoptera: Noctuidae) to Bacillus thuringiensis toxin Cry1Ac in Pakistan. PLoS ONE, 7(10):e47309. [doi:10.1371/journal.pone.0047309]
Cao, J., Zhao, J.Z., Tang, J.D., et al., 2002. Broccoli plants with pyramided cry1Ac and cry1C Bt genes control diamondback moths resistant to Cry1A and Cry1C proteins. Theor. Appl. Genet., 105(2–3):258–264. [doi:10.1007/s00122-002-0942-0]
Dhurua, S., Gujar, G.T., 2011. Field-evolved resistance to Bt toxin Cry1Ac in the pink bollworm, Pectinophora gossypiella (Saunders) (Lepidoptera: Gelechiidae), from India. Pest Manag. Sci., 67(8):898–903. [doi:10.1002/ps.2127]
Ferré, J., Real, M.D., van Rie, J., et al., 1991. Resistance to the Bacillus thuringiensis bioinsecticide in a field population of Plutella xylostella is due to a change in a midgut membrane receptor. PNAS, 88(12):5119–5123. [doi:10.1073/pnas.88.12.5119]
Gassmann, A.J., Petzold-Maxwell, J.L., Keweshan, R.S., et al., 2011. Field-evolved resistance to Bt maize by western corn rootworm. PLoS ONE, 6(7):e22629. [doi:10.1371/journal.pone.0022629]
Halpin, C., 2005. Gene stacking in transgenic plants—the challenge for 21st century plant biotechnology. Plant Biotechnol. J., 3(2):141–155. [doi:10.1111/j.1467-7652.2004.00113.x]
Hiei, Y., Ohta, S., Komari, T., et al., 1994. Efficient transformation of rice (Oryza sativa L.) mediated by Agrobacterium and sequence analysis of the boundaries of the T-DNA. Plant J., 6(2):271–282. [doi:10.1046/j.1365-313X.1994.6020271.x]
James, C., 2013. Global Status of Commercialized Biotech/GM Crops: 2013. ISAAA Brief No. 46. International Service for the Acquisition of Agri-biotech Applications (ISAAA), Ithaca, NY, USA, p.315.
Kruger, M., Rensburg, J.R.J.V., Berg, J.V.D., 2011. Resistance to Bt maize in Busseola fusca (Lepidoptera: Noctuidae) from Vaalharts, South Africa. Environ. Entomol., 40(2): 477–483. [doi:10.1603/EN09220]
Li, J., Yu, H., Zhang, F.Z., et al., 2013. A built-in strategy to mitigate transgene spreading from genetically modified corn. PLoS ONE, 8(12):e81645 [doi:10.1371/journal.pone.0081645]
Liu, Y.G., Mitsukawa, N., Oosumi, T., et al., 1995. Efficient isolation and mapping of Arabidopsis thaliana T-DNA insert junctions by thermal asymmetric interlaced PCR. Plant J., 8(3):457–463. [doi:10.1046/j.1365-313X.1995.08030457.x]
Ruiz de Escudero, I., Estela, A., Porcar, M., et al., 2006. Molecular and insecticidal characterization of a Cry1I protein toxic to insects of the families Noctuidae, Tortricidae, Plutellidae, and Chrysomelidae. Appl. Environ. Microbiol., 72(7):4796–4804. [doi:10.1128/AEM.02861-05]
Sambrock, J., Russel, D.W., 2001. Molecular Cloning: A Laboratory Manual, 3rd Ed. Cold Spring Harbor Laboratory Press, New York.
Storer, N.P., Babcock, J.M., Schlenz, M., et al., 2010. Discovery and characterization of field resistance to Bt maize: Spodoptera frugiperda (Lepidoptera: Noctuidae) in Puerto Rico. J. Econ. Entomol., 103(4):1031–1038. [doi:10.1603/EC10040]
Wang, P., Zhao, J.Z., Rodrigo-Simon, A., et al., 2006. Mechanism of resistance to Bacillus thuringiensis toxin Cry1Ac in a greenhouse population of cabbage looper, Trichoplusia ni. Appl. Environ. Microbiol., 73(4):1199–1207. [doi:10.1128/AEM.01834-06]
Yang, Y., Xu, H., Zheng, X., et al., 2012. Susceptibility and selectivity of Cnaphalocrocis medinalis (Lepidoptera: Pyralidae) to different Cry toxins. J. Econ. Entomol., 105(6):2122–2128. [doi:10.1603/EC12236]
Zhang, H.N., Tian, W., Zhao, J., et al., 2012. Diverse genetic basis of field-evolved resistance to Bt cotton in cotton bollworm from China. PNAS, 109(26):10275–10280. [doi:10.1073/pnas.1200156109]
Zhang, Q., Yu, H., Zhang, F.Z., 2013. Expression and purification of recombinant human serum albumin from selectively terminable transgenic rice. J. Zhejiang Univ.-Sci. B (Biomed. & Biotechnol.), 14(10):867–874. [doi:10.1631/jzus.B1300090]
Zhao, J.Z., Cao, J., Li, Y.X., et al., 2003. Transgenic plants expressing two Bacillus thuringiensis toxins delay insect resistance evolution. Nat. Biotechnol., 21(12):1493–1497. [doi:10.1038/nbt907]
Zhao, Q., Liu, M., Tan, M., et al., 2014. Expression of Cry1Ab and Cry2Ab by a polycistronic transgene with a selfcleavage peptide in rice. PLoS ONE, 9(10):e110006. [doi:10.1371/journal.pone.0110006]
Author information
Authors and Affiliations
Corresponding author
Additional information
Project supported by the National Natural Science Foundation of China (No. 31321063)
ORCID: Qi-chao ZHAO, http://orcid.org/0000-0001-5975-4489
Rights and permissions
About this article
Cite this article
Zhao, Qc., Liu, Mh., Zhang, Xw. et al. Generation of insect-resistant and glyphosate-tolerant rice by introduction of a T-DNA containing two Bt insecticidal genes and an EPSPS gene. J. Zhejiang Univ. Sci. B 16, 824–831 (2015). https://doi.org/10.1631/jzus.B1500056
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1631/jzus.B1500056