Abstract
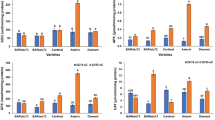
Plants encounter a variety of stresses in natural environments. One-year-old pot-grown trees of pear (Pyrus pyrifolia Nakai cv. Cuiguan and Wonhwang) were exposed to two heat stress regimes. Under constant short-term heat stress, chloroplasts and mitochondria were visibly damaged. Relative chlorophyll content and maximum photochemical efficiency of photosystem II were significantly decreased, which indicated that the leaf photosynthetic capability declined. Under chronic heat stress, mesophyll cell ultrastructure was not obviously damaged, but leaf photosynthetic capability was still restrained. As chronic heat stress was a simulation of the natural environment in summer, further study of the responses under this stress regime was undertaken. Ascorbate peroxidase (APX) activity was increased in ‘Cuiguan’, but not in ‘Wonhwang’. Inducible expression of PpAPX genes in the cytoplasm, chloroplasts and peroxisomes was consistent with increased APX activity in ‘Cuiguan’, whereas only weak induction of PpAPX genes was observed in ‘Wonhwang’. The isoenzymes cytosolic APX1 (cAPX1) and stromal APX (sAPX) were confirmed to be localized in the cytoplasm and chloroplasts, respectively.
Similar content being viewed by others
References
Apel, K., Hirt, H., 2004. Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol., 55(1):373–399. [doi:10.1146/annurev.arplant.55.031903.141701]
Aro, E.M., Suorsa, M., Rokka, A., Allahverdiyeva, Y., Paakkarinen, V., Saleem, A., Battchikova, N., Rintamäki, E., 2005. Dynamics of photosystem II: a proteomic approach to thylakoid protein complexes. J. Exp. Bot., 56(411):347–356. [doi:10.1093/jxb/eri041]
Asada, K., 1992. Ascorbate peroxidase—a hydrogen peroxide-scavenging enzyme in plants. Physiol. Plantarum, 85(2): 235–241. [doi:10.1111/j.1399-3054.1992.tb04728.x]
Asada, K., 1999. The water-water cycle in chloroplasts: scavenging of active oxygen and dissipation of excess photons. Annu. Rev. Plant Physiol. Plant Mol. Biol., 50(1):601–639. [doi:10.1146/annurev.arplant.50.1.601]
Asada, K., 2006. Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiol., 141(2):391–396. [doi:10.1104/pp.106.082040]
Bienert, G.P., Møller, A.L.B., Kristiansen, K.A., Schulz, A., Møller, I.M., Schjoerring, J.K., Jahn, T.P., 2007. Specific aquaporins facilitate the diffusion of hydrogen peroxide across membranes. J. Biol. Chem., 282(2):1183–1192. [doi:10.1074/jbc.M603761200]
Bohnert, H.J., Nelson, D.E., Jensen, R.G., 1995. Adaptations to environment stresses. Plant Cell, 7(7):1099–1111. [doi:10. 2307/3870060]
Chang, C.C.C., Ball, L., Fryer, M.J., Baker, N.R., Karpinski, S., Mullineaux, P.M., 2004. Induction of ascorbate peroxidase 2 expression in wounded Arabidopsis leaves does not involve known wound-signalling pathways but is associated with changes in photosynthesis. Plant J., 38(3):499–511. [doi:10.1111/j.1365-313X.2004.02066.x]
Chew, O., Whelan, J., Millar, A.H., 2003. Molecular definition of the ascorbate-glutathione cycle in Arabidopsis mitochondria reveals dual targeting of antioxidant defenses in plants. J. Biol. Chem., 278(47):46869–46877. [doi:10.1074/jbc.M307525200]
Crafts-Brandner, S.J., Salvucci, M.E., 2002. Sensitivity of photosynthesis in a C4 plant, maize, to heat stress. Plant Physiol., 129(4):1773–1780. [doi:10.1104/pp.002170]
Dat, J., Vandenabeele, S., Vranová, E., van Montagu, M., Inzé, D., van Breusegem, F., 2000. Dual action of the active oxygen species during plant stress responses. Cell. Mol. Life Sci., 57(5):779–795. [doi:10.1007/s000180050041]
Davletova, S., Rizhsky, L., Liang, H., Shengqiang, Z., Oliver, D.J., Coutu, J., Shulaev, V., Schlauch, K., Mittler, R., 2005. Cytosolic ascorbate peroxidase 1 is a central component of the reactive oxygen gene network of Arabidopsis. Plant Cell, 17(1):268–281. [doi:10.1105/tpc.104.026971]
Foyer, C.H., Lelandais, M., Kunert, K.J., 1994. Photooxidative stress in plants. Physiol. Plantarum, 92(4):696–717. [doi:10.1111/j.1399-3054.1994.tb03042.x]
Fridovich, I., 1998. Oxygen toxicity: a radical explanation. J. Exp. Biol., 201(8):1203–1209.
Fryer, M.J., Ball, L., Oxborough, K., Karpinski, S., Mullineaux, P.M., Baker, N.R., 2003. Control of ascorbate peroxidase 2 expression by hydrogen peroxide and leaf water status during excess light stress reveals a functional organisation of Arabidopsis leaves. Plant J., 33(4):691–705. [doi:10.1046/j.1365-313X.2003.01656.x]
Glenn, D.M., Prado, E., Erez, A., Mcferson, J., Puterka, G.J., 2002. A reflective, processed-kaolin particle film affects fruit temperature, radiation reflection, and solar injury in apple. J. Am. Soc. Hortic. Sci., 127(2):188–193.
Havaux, M., 1993. Rapid photosynthetic adaptation to heat stress triggered in potato leaves by moderately elevated temperatures. Plant Cell Environ., 16(4):461–467. [doi:10.1111/j.1365-3040.1993.tb00893.x]
Heath, R.L., Packer, L., 1968. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys., 125(1):189–198. [doi:10.1016/0003-9861(68)90654-1]
Huang, X.Z., Chen, Y.T., Lei, Y., Cai, S.H., Chen, X.M., 2010. Causes and control strategies of a large number of early falling leaves of pear in Fujian. Chin. Agric. Sci. Bull., 26(2):91–95 (in Chinese).
Ishikawa, T., Shigeoka, S., 2008. Recent advances in ascorbate biosynthesis and the physiological significance of ascorbate peroxidase in photosynthesizing organisms. Biosci. Biotechnol. Biochem., 72(5):1143–1154. [doi:10.1271/bbb.80062]
Ishikawa, T., Yoshimura, K., Tamoi, M., Takeda, T., Shigeoka, S., 1997. Alternative mRNA splicing of 3′-terminal exons generates ascorbate peroxidase isoenzymes in spinach (Spinacia oleracea) chloroplasts. Biochem. J., 328(Pt 3): 795–800.
Ishikawa, T., Yoshimura, K., Sakai, K., Tamoi, M., Takeda, T., Shigeoka, S., 1998. Molecular characterization and physiological role of a glyoxysome-bound ascorbate peroxidase from spinach. Plant Cell Physiol., 39(1):23–34. [doi:10.1093/oxfordjournals.pcp.a029285]
Ji, W.W., Qiu, C.H., Jiao, Y., Guo, Y.P., Teng, Y.W., 2012. Effects of high temperature and strong light on photosynthesis, D1 protein, and the Deg1 protease in pear (Pyrus pyrifolia) leaves. J. Fruit Sci., 29(5):794–799.
Jimenez, A., Hernandez, J.A., Del Rio, L.A., Sevilla, F., 1997. Evidence for the presence of the ascorbate-glutathione cycle in mitochondria and peroxisomes of pea leaves. Plant Physiol., 114(1):275–284. [doi:10.1104/pp.114.1.275]
Karpinski, S., Reynolds, H., Karpinska, B., Wingsle, G., Creissen, G., Mullineaux, P., 1999. Systemic signaling and acclimation in response to excess excitation energy in Arabidopsis. Science, 284(5414):654–657. [doi:10.1126/science.284.5414.654]
Kim, M.D., Kim, Y.H., Kwon, S.Y., Yun, D.J., Kwak, S.S., Lee, H.S., 2010. Enhanced tolerance to methyl viologen-induced oxidative stress and high temperature in transgenic potato plants overexpressing the CuZnSOD, APX and NDPK2 genes. Physiol. Plant., 140(2):153–162. [doi:10.1111/j.1399-3054.2010.01392.x]
Kitajima, S., Tomizawa, K.I., Shigeoka, S., Yokota, A., 2006. An inserted loop region of stromal ascorbate peroxidase is involved in its hydrogen peroxide-mediated inactivation. FEBS J., 273(12):2704–2710. [doi:10.1111/j.1742-4658.2006.05286.x]
Kratsch, H.A., Wise, R.R., 2000. The ultrastructure of chilling stress. Plant Cell Environ., 23(4):337–350. [doi:10.1046/j.1365-3040.2000.00560.x]
Krause, G., Weis, E., 1984. Chlorophyll fluorescence as a tool in plant physiology. Photosynth. Res., 5(2):139–157. [doi:10.1007/BF00028527]
Law, R.D., Crafts-Brandner, S.J., 1999. Inhibition and acclimation of photosynthesis to heat stress is closely correlated with activation of ribulose-1,5-bisphosphate carboxylase/oxygenase. Plant Physiol., 120(1):173–182. [doi:10.1104/pp.120.1.173]
Li, P.M., Cheng, L.L., 2009. The elevated anthocyanin level in the shaded peel of ‘anjou’ pear enhances its tolerance to high temperature under high light. Plant Sci., 177(5): 418–426. [doi:10.1016/j.plantsci.2009.07.005]
Liu, G., Li, W., Zheng, P., Xu, T., Chen, L., Liu, D., Hussain, S., Teng, Y., 2012. Transcriptomic analysis of ‘suli’ pear (Pyrus pyrifolia white pear group) buds during the dormancy by RNA-Seq. BMC Genomics, 13(1):700. [doi:10.1186/1471-2164-13-700]
Livak, K.J., Schmittgen, T.D., 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods, 25(4):402–408. [doi:10.1006/meth.2001.1262]
Ma, Y.H., Ma, F.W., Zhang, J.K., Li, M.J., Wang, Y.H., Liang, D., 2008. Effects of high temperature on activities and gene expression of enzymes involved in ascorbate-glutathione cycle in apple leaves. Plant Sci., 175(6): 761–766. [doi:10.1016/j.plantsci.2008.07.010]
Martinez, S.E., Huang, D., Szczepaniak, A., Cramer, W.A., Smith, J.L., 1994. Crystal structure of chloroplast cytochrome reveals a novel cytochrome fold and unexpected heme ligation. Structure, 2(2):95–105. [doi:10.1016/S0969-2126(00)00012-5]
Mittler, R., 2002. Oxidative stress antioxidantandstress tolerance. Trends Plant Sci., 7(9):405–410. [doi:10.1016/S1360-1385(02)02312-9]
Mittler, R., Zilinskas, B.A., 1992. Molecular cloning and characterization of a gene encoding pea cytosolic ascorbate peroxidase. J. Biol. Chem., 267(30):21802–21807.
Mittler, R., Vanderauwera, S., Gollery, M., van Breusegem, F., 2004. Reactive oxygen gene network of plants. Trends Plant Sci., 9(10):490–498. [doi:10.1016/j.tplants.2004.08.009]
Miyake, C., Asada, K., 1996. Inactivation mechanism of ascorbate peroxidase at low concentrations of ascorbate; hydrogen peroxide decomposes compound I of ascorbate peroxidase. Plant Cell Physiol., 37(4):423–430. [doi:10. 1093/oxfordjournals.pcp.a028963]
Mullineaux, P.M., 2006. Spatial dependence for hydrogen peroxide-directed signaling in light-stressed plants. Plant Physiol., 141(2):346–350. [doi:10.1104/pp.106.078162]
Najami, N., Janda, T., Barriah, W., Kayam, G., Tal, M., Guy, M., Volokita, M., 2008. Ascorbate peroxidase gene family in tomato: its identification and characterization. Mol. Genet. Genomics, 279(2):171–182. [doi:10.1007/s00438-007-0305-2]
Nakano, Y., Asada, K., 1981. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol., 22(5):867–880.
Neill, S., Desikan, R., Hancock, J., 2002. Hydrogen peroxide signalling. Curr. Opin. Plant Biol., 5(5):388–395. [doi:10.1016/S1369-5266(02)00282-0]
Panchuk, I.I., Volkov, R.A., Schöffl, F., 2002. Heat stress- and heat shock transcription factor-dependent expression and activity of ascorbate peroxidase in Arabidopsis. Plant Physiol., 129(2):838–853. [doi:10.1104/pp.001362]
Santos, M., Gousseau, H., Lister, C., Foyer, C., Creissen, G., Mullineaux, P., 1996. Cytosolic ascorbate peroxidase from Arabidopsis thaliana L. is encoded by a small multigene family. Planta, 198(1):64–69. [doi:10.1007/BF00197587]
Sato, Y., Murakami, T., Funatsuki, H., Matsuba, S., Saruyama, H., Tanida, M., 2001. Heat shock-mediated APX gene expression and protection against chilling injury in rice seedlings. J. Exp. Bot., 52(354):145–151. [doi:10.1093/jexbot/52.354.145]
Sečenji, M., Hideg, E., Bebes, A., Györgyey, J., 2009. Transcriptional differences in gene families of the ascorbate-glutathione cycle in wheat during mild water deficit. Plant Cell Rep., 29(1):37–50. [doi:10.1007/s00299-009-0796-x]
Shigeoka, S., Ishikawa, T., Tamoi, M., Miyagawa, Y., Takeda, T., Yabuta, Y., Yoshimura, K., 2002. Regulation and function of ascorbate peroxidase isoenzymes. J. Exp. Bot., 53(372):1305–1319. [doi:10.1093/jexbot/53.372.1305]
Sparkes, I.A., Runions, J., Kearns, A., Hawes, C., 2006. Rapid, transient expression of fluorescent fusion proteins in tobacco plants and generation of stably transformed plants. Nat. Protoc., 1(4):2019–2025. [doi:10.1038/nprot.2006.286]
Teixeira, F., Menezes-Benavente, L., Margis, R., Margis-Pinheiro, M., 2004. Analysis of the molecular evolutionary history of the ascorbate peroxidase gene family: inferences from the rice genome. J. Mol. Evol., 59(6):761–770. [doi:10.1007/s00239-004-2666-z]
Teixeira, F., Menezes-Benavente, L., Galvão, V., Margis, R., Margis-Pinheiro, M., 2006. Rice ascorbate peroxidase gene family encodes functionally diverse isoforms localized in different subcellular compartments. Planta, 224(2):300–314. [doi:10.1007/s00425-005-0214-8]
Vandenabeele, S., van der Kelen, K., Dat, J., Gadjev, I., Boonefaes, T., Morsa, S., Rottiers, P., Slooten, L., van Montagu, M., Zabeau, M., et al., 2003. A comprehensive analysis of hydrogen peroxide-induced gene expression in tobacco. PNAS, 100(26):16113–16118. [doi:10.1073/pnas.2136610100]
Vanderauwera, S., Suzuki, N., Miller, G., van de Cotte, B., Morsa, S., Ravanat, J.L., Hegie, A., Triantaphylidès, C., Shulaev, V., van Montagu, M.C.E., et al., 2011. Extranuclear protection of chromosomal DNA from oxidative stress. PNAS, 108(4):1711–1716. [doi:10.1073/pnas.1018359108]
Volkov, R., Panchuk, I., Mullineaux, P., Schoffl, F., 2006. Heat stress-induced H2O2 is required for effective expression of heat shock genes in Arabidopsis. Plant Mol. Biol., 61(4–5):733–746. [doi:10.1007/s11103-006-0045-4]
Wang, J., Ou, Y., Wu, Z., Dai, L.Z., Liu, S.W., 2011. Effects of high temperature stress on physiological indicators, early defoliation of early-maturing pear. Southwest China J. Agric. Sci., 24(2):546–551 (in Chinese).
Yamamoto, Y., Aminaka, R., Yoshioka, M., Khatoon, M., Komayama, K., Takenaka, D., Yamashita, A., Nijo, N., Inagawa, K., Morita, N., et al., 2008. Quality control of photosystem II: impact of light and heat stresses. Photosynth. Res., 98(1–3):589–608. [doi:10.1007/s11120-008-9372-4]
Yoshimura, K., Yabuta, Y., Ishikawa, T., Shigeoka, S., 2000. Expression of spinach ascorbate peroxidase isoenzymes in response to oxidative stresses. Plant Physiol., 123(1): 223–234. [doi:10.1104/pp.123.1.223]
Yu, B., Zhang, D., Huang, C., Qian, M., Zheng, X., Teng, Y., Su, J., Shu, Q., 2012. Isolation of anthocyanin biosynthetic genes in red Chinese sand pear (Pyrus pyrifolia Nakai) and their expression as affected by organ/tissue, cultivar, bagging and fruit side. Sci. Hort., 136:29–37. [doi:10.1016/j.scienta.2011.12.026]
Author information
Authors and Affiliations
Corresponding author
Additional information
Project (No. nycytx-29) supported by the Earmarked Fund for the Modern Agro-industry Technology Research System, China
Rights and permissions
About this article
Cite this article
Liu, Df., Zhang, D., Liu, Gq. et al. Influence of heat stress on leaf ultrastructure, photosynthetic performance, and ascorbate peroxidase gene expression of two pear cultivars (Pyrus pyrifolia). J. Zhejiang Univ. Sci. B 14, 1070–1083 (2013). https://doi.org/10.1631/jzus.B1300094
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1631/jzus.B1300094