Abstract
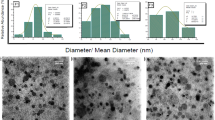
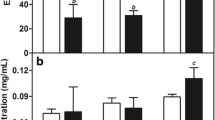
Docetaxel (DTX), as a member of taxoid family, has been widely used in the treatment of cancers. The present study prepared pH-sensitive DTX-loaded liposomes (DTX-Lips) by thin-film dispersion method and various physico-chemical and morphological properties were examined. The pH sensitivity of in vitro DTX release and the in vivo pharmacokinetics and tissue distribution using Kunming mice were also investigated. The mean particle size and zeta potential of DTX liposomes were (277±2) nm and (−32.60±0.26) mV, respectively. Additionally, in vitro drug release study showed that the cumulative release rate was 1.3 times more at pH 5.0 than at pH 7.4, suggesting a pH-dependent release ability of DTX-Lips. Pharmacokinetic and pharmaceutical studies in comparison with Duopafei® showed that the half-time period (t 1/2) and area under the curve (AUC) of DTX-Lips in mouse plasma were 1.8 times longer and 2.6 times higher, respectively, and that DTX-Lips selectively accumulated in macrophage-rich organs such as liver and spleen. These results together suggest that the DTX-Lips could be a promising formulation for the clinical administration of DTX.
Similar content being viewed by others
References
Baker, J., Ajani, J., Scotté, F., Winther, D., Martin, M., Aapro, M.S., von Minckwitz, G., 2009. Docetaxel-related side effects and their management. Eur. J. Oncol. Nurs., 13(1):49–59. [doi:10.1016/j.ejon.2008.10.003]
Bissery, M.C., 1995. Preclinical pharmacology of docetaxel. Eur. J. Cancer, 31(S4):S1–S6. [doi:10.1016/0959-8049 (95)00357-O]
Chu, C.J., Dijkstra, J., Lai, M.Z., Hong, K., Szoka, F.C., 1990. Efficiency of cytoplasmic delivery by pH-sensitive liposomes to cells in culture. Pharm. Res., 7(8):824–834. [doi:10.1023/A:1015908831507]
Connor, J., Huang, L., 1985. Efficient cytoplasmic delivery of a fluorescent dye by pH-sensitive immunoliposomes. J. Cell Biol., 101(2):582–589. [doi:10.1083/jcb.101.2.582]
Ducat, E., Deprez, J., Peulen, O., Evrard, B., Piel, G., 2010. Cellular uptake of long-circulating pH sensitive liposomes: evaluation of the liposome and its encapsulated material penetration in cancer cells. Drug Discov. Today, 15(23-24):1083. [doi:10.1016/j.drudis.2010.09.362]
Engels, F.K., Sparreboom, A., Mathot, R.A., Verweij, J., 2005. Potential for improvement of docetaxel-based chemotherapy: a pharmacological review. Br. J. Cancer, 93(2):173–177. [doi:10.1038/sj.bjc.6602698]
Friedenberg, W.R., Graham, D., Greipp, P., Blood, E., Winston, R.D., 2003. The treatment of multiple myeloma with docetaxel (an ECOG study). Leuk. Res., 27(8):751–754. [doi:10.1016/S0145-2126(02)00344-2]
Gelmon, K., 1994. The taxoids: paclitaxel and docetaxel. Lancet, 344(8932):1267–1272. [doi:10.1016/S0140-6736 (94)90754-4]
Grant, D.S., Williams, T.L., Zahaczewsky, M., Dicker, A.P., 2003. Comparison of antiangiogenic activities using paclitaxel (taxol) and docetaxel (taxotere). Int. J. Cancer, 104(1):121–129. [doi:10.1002/ijc.10907]
Hait, W.N., Rublin, E., Alli, E., Goodin, S., 2007. Tubulin targeting agents. Update on Cancer Therapeutics., 2(1):1–18. [doi:10.1016/j.uct.2006.10.001].
Hanauske, A.R., Depenbrock, H., Shirvani, D., Rastetter, J., 1994. Effects of the microtubule-disturbing agents docetaxel (Taxotere®), vinblastine and vincristine on epidermal growth factor-receptor binding of human breast cancer cell lines in vitro. Eur. J. Cancer, 30(11):1688–1694. [doi:10.1016/0959-8049(94)00338-6]
Herbst, R.S., Khuri, F.R., 2003. Mode of action of docetaxel-a basis for combination with novel anticancer agents. Cancer Treat. Rev., 29(5):407–415. [doi:10.1016/S0305-7372(03)00097-5]
Hiraka, K., Kanehisa, M., Tamai, M., Asayama, S., Nagaoka, S., Oyaizu, K., Yuasa, M., Kawakami, H., 2008. Preparation of pH-sensitive liposomes retaining SOD mimic and their anticancer effect. Colloids Surf. B Biointerfaces, 67(1):54–58. [doi:10.1016/j.colsurfb.2008.07.014]
Immordino, M.L., Brusa, P., Arpicco, S., Stella, B., Dosio, F., Catte, L., 2003. Preparation, characterization, cytotoxicity and pharmacokinetics of liposomes containing docetaxel. J. Control. Release, 91(3):417–429. [doi:10.1016/S0168-3659(03)00271-2]
Izquierdo, M.A., García, M., Pontón, J.L., Martínez, M., Valentí, V., Navarro, M., Gil, M., Cardenal, F., Mesía, R., Pérez, X., Salazar, R., Germà-Lluch, J.R., 2006. A phase I clinical and pharmacokinetic study of paclitaxel and docetaxel given in combination in patients with solid tumours. Eur. J. Cancer, 42(12):1789–1796. [doi:10.1016/j.ejca.2005.10.031]
Kale, A.A., Torchilin, V.P., 2010. Environment-responsive multifunctional liposomes. Methods Mol. Biol., 605: 213–242. [doi:10.1007/978-1-60327-360-2_15]
Li, J., Yu, H., Li, S., Wang, G.J., 2010. Enhanced distribution and extended elimination of glycyrrhetinic acid in mice liver by mPEG-PLA modified (mPEGylated) liposome. J. Pharm. Biomed. Anal., 51(5):1147–1153. [doi:10.1016/j. jpba.2009.11.005]
Lian, T., Ho, R.J., 2001. Trends and developments in liposome drug delivery systems. J. Pharm. Sci., 90(6):667–680. [doi:10.1002/jps.1023]
Maurer, N., Fenske, D.B., Cullis, P.R., 2001. Developments in liposomal drug delivery Systems. Expert. Opin. Biol. Ther., 1(6):923–947. [doi:10.1517/14712598.1.6.923]
Muthu, M.S., Singh, S., 2009. Targeted nanomedicines: effective treatment modalities for cancer, AIDS and brain disorders. Nanomedicine, 4(1):105–118. [doi:10.2217/17435889.4.1.105]
Parveen, S., Misra, R., Sahoo, S.K., 2012. Nanoparticles: a boon to drug delivery, therapeutics, diagnostics and imaging. Nanomedicine, 8(2):147–166. [doi:10.1016/j. nano.2011.05.016]
Philippot, J.R., Mutaftschiev, S., Liautard. J.P., 1985. Extemporaneous preparation of large unilamellar liposomes. Biochim. Biophys. Acta., 821(1):79–84. [doi:10.1016/0005-2736(85)90156-7]
Posner, M.R, Lefebvre, J.L., 2003. Docetaxel induction therapy in locally advanced squamous cell carcinoma of the head and neck. Br. J. Cancer, 88(1):11–17. [doi:10. 1038/sj.bjc.6600685]
Rowinsky, E.K., 1997. The development and clinical utility of the taxane class of antimicrotubule chemotherapy agents. Annu. Rev. Med., 48:353-374. [doi:10.1146/annurev. med.48.1.353].
Sánchez, M., Aranda, F.J., Teruel, J.A., Ortiz, A., 2011. New pH-sensitive liposomes containing phosphatidylethanolamine and a bacterial dirhamnolipid. Chem. Phys. Lipids, 164(1):16–23. [doi:10.1016/j.chemphyslip.20 10.09.008]
Straubinger, R.M., Düzgünes, N., Papahadjopoulos, D., 1985. pH-sensitive liposomes mediate cytoplasmic delivery of encapsulated macromolecules, FEBS Lett., 179(1):148–154. [doi:10.1016/0014-5793(85)80210-6]
Torchilin, V.P., 2005. Recent advances with liposomes as pharmaceutical carriers. Nat. Rev. Drug Discov., 4(2):145–160. [doi:10.1038/nrd1632]
Torchilin, V.P., Zhou, F., Huang, L., 1993. pH-sensitive liposomes. J. Liposome Res., 3(2):201–255. [doi:10.3109/08982109309148213]
Torchilin, V.P., Lukyanov, A.N., Klibanov, A.L., Omelyanenko, V.G., 1992. Interaction between oleic acid-containing pH-sensitive and plain liposomes. Fluorescent spectroscopy studies. FEBS Lett., 305(3):185–188. [doi:10. 1016/0014-5793(92)80663-2]
Tosi, G., Vergoni, A.V., Ruozi, B., Bondioli, L., Badiali, L., Rivasi, F., Costantino, L., Forni, F., Vandelli, M.A., 2010. Sialic acid and glycopeptides conjugated PLGA nanoparticles for central nervous system targeting: in vivo pharmacological evidence and biodistribution. J. Control. Release, 145(1):49–57. [doi:10.1016/j.jconrel. 2010.03.008]
Vassilomanolakis, M., Koumakis, G., Barbounis, V., Demiri, M., Panopoulos, C., Chrissohoou, M., Apostolikas, N., Efremidis, A.P., 2005. First-line chemotherapy with docetaxel and cisplatin in metastatic breast cancer. Breast, 14(2):136–141. [doi:10.1016/j.breast.2004.08.017]
Yang, F., Jin, C., Jiang, Y., Li, J., Di, Y., Ni, Q., Fu, D., 2011. Liposome based delivery systems in pancreatic cancer treatment: from bench to bedside. Cancer Treat. Rev., 37(8):633–642. [doi:10.1016/j.ctrv.2011.01.006]
Yuba, E., Kojima, C., Harada, A., Tana, Watarai, S., Kono., K., 2010. pH-sensitive fusogenic polymer-modified liposomes as a carrierof antigenic proteins for activation of cellular immunity. Biomaterials, 31(5):943–951. [doi:10.1016/j.biomaterials.2009.10.006]
Zhang, P., Ling, G., Pan, X., Sun, J., Zhang, T., Pu, X., Yin, S., He, Z., 2012. Novel nanostructured lipid-dextran sulfate hybrid carriers overcome tumor multidrug resistance of mitoxantrone hydrochloride. Nanomedicine, 8(2):185–193. [doi:10.1016/j.nano.2011.06.007]
Zhao, L., Wei, Y.M., Zhong, X.D., Liang, Y., Zhang, X.M., Li, W., Li, B.B., Wang, Y., Yu, Y., 2009. PK and tissue distribution of docetaxel in rabbits after i.v. administration of liposomal and injectable formulations. J. Pharm. Biomed. Anal., 49(4):989–996. [doi:10.1016/j.jpba.2009.01.016]
Zignani, M., Drummond, D.C., Meyer, O., Hong, K., Leroux, J.C., 2000. In vitro characterization of a novel polymeric-based pH-sensitive liposome system. Biochim. Biophs. Acta, 1463(2):383–394. [doi:10.1016/S0005-2736(99)00234-5]
Author information
Authors and Affiliations
Corresponding author
Additional information
Project supported by the Independent Innovation Foundation of Shandong University, China (No. 2010TS041) and the Shandong Provincial Special Funds for Postdoctoral Innovative Projects, China (No. 201003069)
Rights and permissions
About this article
Cite this article
Zhang, H., Li, Ry., Lu, X. et al. Docetaxel-loaded liposomes: preparation, pH sensitivity, Pharmacokinetics, and tissue distribution. J. Zhejiang Univ. Sci. B 13, 981–989 (2012). https://doi.org/10.1631/jzus.B1200098
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1631/jzus.B1200098