Abstract
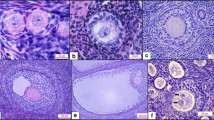
There is a lack of appropriate classification criteria for the determination of atretic follicles in guinea pigs. In the present study, new criteria were established based on the latest morphologic criteria for cell death proposed by the Nomenclature Committee on Cell Death (NCCD) in 2009. Ovaries of guinea pigs were sampled on different stages of estrous cycle, and the morphologic observations of atretic follicles were investigated in serial sections. The results showed that the process of follicular atresia could be classified into four continuous stages: (1) the granulosa layer became loose, and some apoptotic bodies began to appear; (2) the granulosa cells were massively eliminated; (3) the theca interna cells differentiated; and (4) the residual follicular cells degenerated. In addition, the examination revealed that these morphologic criteria were accurate and feasible. In conclusion, this study provides new criteria for the classification of atretic follicles in guinea pigs, and this knowledge can inform future research in the area.
Similar content being viewed by others
References
Bland, K.P., 1980. Biphasic follicular growth in the guinea-pig oestrous cycle. J. Reprod. Fertil., 60(1):73–76. [doi:10.1530/jrf.0.0600073]
Byskov, A.G., 1974. Cell kinetic studies of follicular atresia in the mouse ovary. J. Reprod. Fertil., 37(2):277–285. [doi:10.1530/jrf.0.0370277]
Cui, Y., Yu, S.J., 1999. Ovarian morphology and follicular systems in yaks of different ages. Vet. J., 157(2):197–205. [doi:10.1053/tvjl.1998.0282]
Fortune, J.E., 1994. Ovarian follicular growth and development in mammals. Biol. Reprod., 50(2):225–232. [doi:10.1095/biolreprod50.2.225]
Galluzzi, L., Maiuri, M.C., Vitale, I., Zischka, H., Castedo, M., Zitvogel, L., Kroemer, G., 2007. Cell death modalities: classification and pathophysiological implications. Cell Death Differ., 14(7):1237–1243. [doi:10.1038/sj.cdd.4402148]
Garcia, R., Ballesteros, L.M., Hernandez-Perez, O., Rosales, A.M., Espinosa, R., Soto, H., Diaz de Leon, L., Rosado, A., 1997. Metalloproteinase activity during growth, maturation and atresia in the ovarian follicles of the goat. Anim. Reprod. Sci., 47(3):211–228. [doi:10.1016/S0378-4320(96)01637-5]
Garris, D.R., Mitchell, J.A., 1979. Intrauterine oxygen tension during the estrous cycle in the guinea pig: its relation to uterine blood volume and plasma estrogen and progesterone levels. Biol. Reprod., 21(1):149–159. [doi:10.1095/biolreprod21.1.149]
Hirshfield, A.N., 1988. Size-frequency analysis of atresia in cycling rats. Biol. Reprod., 38(5):1181–1188. [doi:10.1095/biolreprod38.5.1181]
Hutz, R.J., Bejvan, S.M., Durning, M., Dierschke, D.J., 1990. Changes in follicular populations, in serum estrogen and progesterone, and in ovarian steroid secretion in vitro during the guinea pig estrous cycle. Biol. Reprod., 42(2):266–272. [doi:10.1095/biolreprod42.2.266]
Joshi, H.S., Watson, D.J., Labhsetwar, A.P., 1973. Ovarian secretion of oestradiol, oestrone, 20-dihydroprogesterone and progesterone during the oestrous cycle of the guinea-pig. J. Reprod. Fertil., 35(1):177–181. [doi:10.1530/jrf.0.0350177]
Kasuya, K., 1997. Elimination of apoptotic granulosa cells by intact granulosa cells and macrophages in atretic mature follicles of the guinea pig ovary. Arch. Histol. Cytol., 60(2):175–184. [doi:10.1679/aohc.60.175]
Kerr, J.F., Winterford, C.M., Harmon, B.V., 1994. Apoptosis: its significance in cancer and cancer therapy. Cancer, 73(8):2013–2026. [doi:10.1002/1097-0142(19940415)73:8<2013::AID-CNCR2820730802>3.0.CO;2-J]
Kroemer, G., El-Deiry, W.S., Golstein, P., Peter, M.E., Vaux, D., Vandenabeele, P., Zhivotovsky, B., Blagosklonny, M.V., Malorni, W., Knight, R.A., et al., 2005. Classification of cell death: recommendations of the Nomenclature Committee on Cell Death. Cell Death Differ., 12(Suppl. 2):1463–1467. [doi:10.1038/sj.cdd.4401724]
Kroemer, G., Galluzzi, L., Vandenabeele, P., Abrams, J., Alnemri, E.S., Baehrecke, E.H., Blagosklonny, M.V., El-Deiry, W.S., Golstein, P., Green, D.R., et al., 2009. Classification of cell death: recommendations of the Nomenclature Committee on Cell Death 2009. Cell Death Differ., 16(1):3–11. [doi:10.1038/cdd.2008.150]
Logothetopoulos, J., Dorrington, J., Bailey, D., Stratis, M., 1995. Dynamics of follicular growth and atresia of large follicles during the ovarian cycle of the guinea pig: fate of the degenerating follicles, a quantitative study. Anat. Rec., 243(1):37–48. [doi:10.1002/ar.1092430106]
Norris, M.L., Adams, C.E., 1979. The vaginal smear, mating, egg transport and preimplantation development in a wild guinea-pig, the cuis (Galea musteloides). J. Reprod. Fertil., 55(2):457–461. [doi:10.1530/jrf.0.0550457]
Osman, P., 1985. Rate and course of atresia during follicular development in the adult cyclic rat. J. Reprod. Fertil., 73(1):261–270. [doi:10.1530/jrf.0.0730261]
Shi, F., Ozawa, M., Komura, H., Yang, P., Trewin, A.L., Hutz, R.J., Watanabe, G., Taya, K., 1999. Secretion of ovarian inhibin and its physiologic roles in the regulation of follicle-stimulating hormone secretion during the estrous cycle of the female guinea pig. Biol. Reprod., 60(1):78–84. [doi:10.1095/biolreprod60.1.78]
Shi, F., Ozawa, M., Komura, H., Watanabe, G., Tsonis, C.G., Suzuki, A.K., Taya, K., 2000a. Induction of superovulation by inhibin vaccine in cyclic guinea-pigs. J. Reprod. Fertil., 118(1):1–7. [doi:10.1530/reprod/118.1.1]
Shi, F., Watanabe, G., Trewin, A.L., Hutz, R.J., Taya, K., 2000b. Localization of ovarian inhibin/activin subunits in follicular dominance during the estrous cycle of guinea pigs. Zool. Sci., 17(9):1311–1320. [doi:10.2108/zsj.17.1311]
Sugimoto, M., Manabe, N., Kimura, Y., Myoumoto, A., Imai, Y., Ohno, H., Miyamoto, H., 1998. Ultrastructural changes in granulosa cells in porcine antral follicles undergoing atresia indicate apoptotic cell death. J. Reprod. Dev., 44(1):7–14. [doi:10.1262/jrd.44.7]
Suzuki, O., Koura, M., Noguchi, Y., Takano, K., Yamamoto, Y., Matsuda, J., 2003. Optimization of superovulation induction by human menopausal gonadotropin in guinea pigs based on follicular waves and FSH-receptor homologies. Mol. Reprod. Dev., 64(2):219–225. [doi:10.1002/mrd.10242]
Trewin, A.L., Chaffin, C.L., Watanabe, G., Taya, K., Hutz, R.J., 1998. Cyclic changes in serum follicle-stimulating hormone, luteinizing hormone and inhibin during the guinea pig estrous cycle. J. Reprod. Dev., 44(4):353–357. [doi:10.1262/jrd.44.353]
van der Hoek, K.H., Maddocks, S., Woodhouse, C.M., van Rooijen, N., Robertson, S.A., Norman, R.J., 2000. Intrabursal injection of clodronate liposomes causes macrophage depletion and inhibits ovulation in the mouse ovary. Biol. Reprod., 62(4):1059–1066. [doi:10.1095/biolreprod62.4.1059]
van Kan, C.M., de Vries, J.I., Luchinger, A.B., Mulder, E.J., Taverne, M.A., 2009. Ontogeny of fetal movements in the guinea pig. Physiol. Behav., 98(3):338–344. [doi:10.1016/j.physbeh.2009.06.011]
Wu, R., van der Hoek, K.H., Ryan, N.K., Norman, R.J., Robker, R.L., 2004. Macrophage contributions to ovarian function. Hum. Reprod. Update, 10(2):119–133. [doi:10.1093/humupd/dmh011]
Author information
Authors and Affiliations
Corresponding author
Additional information
Project supported by the National Basic Research Program (973) of China (No. 2007CB947403) and the Open-Ended Fund for the Youth of China Agricultural University-Nanjing Agricultural University (No. NC2008005)
Rights and permissions
About this article
Cite this article
Wang, W., Liu, Hl., Tian, W. et al. Morphologic observation and classification criteria of atretic follicles in guinea pigs. J. Zhejiang Univ. Sci. B 11, 307–314 (2010). https://doi.org/10.1631/jzus.B0900391
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1631/jzus.B0900391