Abstract
This study demonstrated the impacts of the synthesis methods on the textural structures, chemical properties, and Hg0 capture capability of the MnOx system. Compared with the samples synthesized using the precipitation (PR) and hydrothermal (HT) methods, the adsorbent prepared via the sol-gel (SG) technique gave the best performance. At 150 °C, ca. 90% Hg0 removal efficiency was reached after 7.5 h for MnOx prepared by the SG method, ca. 40% higher than that of the other two methods. The specific surface area of the adsorbent synthesized via the SG technique (23 m2/g) was almost double that of the adsorbent prepared by the HT method (12 m2/g) and three times that of the one prepared by the PR method (7 m2/g). The presence of plentiful acid sites from the SG method facilitated the physisorption of Hg0, making more Hg0 available to be oxidized to HgO by the redox sites and thus giving the adsorbent prepared by the SG method the highest Hg0 removal efficiency. The strong oxidative ability accelerated the oxidation of the physically adsorbed Hg0 to HgO, which explained the higher Hg0 removal efficiency of the sample prepared using the HT method than that of the one synthesized by the PR technique. During the whole Hg0 removal cycles, chemisorption dominated, with the initial adsorption stage and the external mass-transfer process playing important roles.
摘要
目的
针对当前单质汞高效脱除难题, 本文旨在探讨不同制备方法对锰氧化物吸附剂单质汞捕集性能的影响, 并通过对吸附剂物理化学性质的研究, 揭示锰氧化物吸附剂的构效关系及脱汞机理。
创新点
1. 揭示锰氧化物吸附剂脱汞性能与物化性质之间的构效关系; 2. 阐明锰氧化物吸附剂表面脱汞机理, 并揭示其中的活性位点。
方法
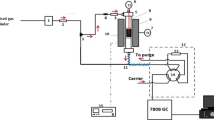
1. 采用常规的沉淀法、水热法及溶胶凝胶法合成锰氧化物吸附剂; 2. 采用微型固定床反应器研究系列锰吸附剂的脱汞性能; 3. 采用物化表征研究系列锰氧化物汞吸附剂的物化特性, 揭示其中的构效关系; 4. 结合动力学计算, 揭示锰氧化物汞吸附剂表面脱汞机理, 并阐明影响吸附剂脱汞性能的关键因素。
结论
1. 与采用沉淀法及水热法制备的汞吸附剂相比, 采用溶胶凝胶法制备的汞吸附剂在150~250 °C的温度区间内具有更为优异的脱汞性能; 2. 采用溶胶凝胶法制备的锰氧化物吸附剂具有丰富的表面酸性位点, 可促进单质汞的吸附, 并为接下来的单质汞氧化步骤提供充足的反应物, 这也是该吸附剂具有优异脱汞性能的主要原因; 3. 脱汞过程中, 初始吸附阶段是主要的速控阶段, 而Mn4+及化学吸附氧为该反应中的主要活性位点。
Similar content being viewed by others
Reference
Chalkidis A, Jampaiah D, Amin MH, et al., 2019a. CeO2-decorated α-MnO2 nanotubes: a highly efficient and regenerable sorbent for elemental mercury removal from natural gas. Langmuir, 35(25):8246–8256. https://doi.org/10.1021/acs.langmuir.9b00835
Chalkidis A, Jampaiah D, Hartley PG, et al., 2019b. Regenerable α-MnO2 nanotubes for elemental mercury removal from natural gas. Fuel Processing Technology, 193:317–327. https://doi.org/10.1016/j.fuproc.2019.05.034
Chen JH, Cao FF, Chen SZ, et al., 2014. Adsorption kinetics of NO on ordered mesoporous carbon (OMC) and cerium-containing OMC (Ce-OMC). Applied Surface Science, 317:26–34. https://doi.org/10.1016/j.apsusc.2014.08.067
Chen JH, Cao FF, Qu RY, et al., 2015. Bimetallic cerium-copper nanoparticles embedded in ordered mesoporous carbons as effective catalysts for the selective catalytic reduction of NO with NH3. Journal of Colloid and Interface Science, 456:66–75. https://doi.org/10.1016/j.jcis.2015.06.001
Fulazzaky MA, Khamidun MH, Omar R, 2013. Understanding of mass transfer resistance for the adsorption of solute onto porous material from the modified mass transfer factor models. Chemical Engineering Journal, 228:1023–1029
Gao X, Jiang Y, Fu YC, et al., 2010. Preparation and characterization of CeO2/TiO2 catalysts for selective catalytic reduction of NO with NH3. Catalysis Communications, 11(5):465–469. https://doi.org/10.1016/j.catcom.2009.11.024
Jia JB, Zhang PY, Chen L, 2016. The effect of morphology of α-MnO2 on catalytic decomposition of gaseous ozone. Catalysis Science & Technology, 6(15):5841–5847. https://doi.org/10.1039/C6CY00301J
Liu CX, Chen L, Chang HZ, et al., 2013. Characterization of CeO2−WO3 catalysts prepared by different methods for selective catalytic reduction of NOx with NH3. Catalysis Communications, 40:145–148. https://doi.org/10.1016/j.catcom.2013.06.017
Liu DJ, Zhang Z, Luo F, et al., 2020a. Elemental mercury capture from simulated flue gas by graphite-phase carbon nitride. Energy & Fuels, 34(6):6851–6861. https://doi.org/10.1021/acs.energyfuels.0c00457
Liu DJ, Li CE, Wu J, et al., 2020b. Novel carbon-based sorbents for elemental mercury removal from gas streams: a review. Chemical Engineering Journal, 391:123514. https://doi.org/10.1016/j.cej.2019.123514
Liu H, Chang L, Liu WJ, et al., 2020. Advances in mercury removal from coal-fired flue gas by mineral adsorbents. Chemical Engineering Journal, 379:122263. https://doi.org/10.1016/j.cej.2019.122263
Liu SL, Ji J, Yu Y, et al., 2018. Facile synthesis of amorphous mesoporous manganese oxides for efficient catalytic decomposition of ozone. Catalysis Science & Technology, 8(16):4264–4273. https://doi.org/10.1039/C8CY01111G
MEP (Ministry of Environmental Protection of the People’s Republic of China), 2011. Emission Standard of Air Pollutants for Thermal Power Plants, GB13223-2011. National Standards of the People’s Republic of China (in Chinese).
Qu RY, Gao X, Cen KF, et al., 2013. Relationship between structure and performance of a novel cerium-niobium binary oxide catalyst for selective catalytic reduction of NO with NH3. Applied Catalysis B: Environmental, 142–143:290–297. https://doi.org/10.1016/j.apcatb.2013.05.035
Ramesh K, Chen LW, Chen FX, et al., 2008. Re-investigating the CO oxidation mechanism over unsupported MnO, Mn2O3 and MnO2 catalysts. Catalysis Today, 131(1–4): 477–482. https://doi.org/10.1016/j.cattod.2007.10.061
Shi YJ, Deng S, Wang HM, et al., 2016. Fe and Co modified vanadium-titanium steel slag as sorbents for elemental mercury adsorption. RSC Advances, 6(19):15999–16009. https://doi.org/10.1039/c5ra26712a
Wang HN, Ma W, Yan JB, et al., 2020. Smart modification of HZSM-5 with manganese species for the removal of mercury. ACS Omega, 5(30):19277–19284. https://doi.org/10.1021/acsomega.0c02877
Wang Z, Liu J, Yang YJ, et al., 2020. Insights into the catalytic behavior of LaMnO3 perovskite for Hg0 oxidation by HCl. Journal of Hazardous Materials, 383:121156. https://doi.org/10.1016/j.jhazmat.2019.121156
Xu HM, Xie JK, Ma YP, et al., 2015. The cooperation of Fe−Sn in a MnOx complex sorbent used for capturing elemental mercury. Fuel, 140:803–809. https://doi.org/10.1016/j.fuel.2014.10.004
Yang LT, Wu J, Li B, et al., 2021. Defective molybdenum disulfide nanosheet for elemental mercury capture in simulated flue gas. Journal of the Energy Institute, 94:120–128. https://doi.org/10.1016/j.joei.2020.11.007
Yang R, Mei CL, Wu XS, et al., 2019. Mn−Cu binary metal oxides with molecular-scale homogeneity for Hg0 removal from coal-fired flue gas. Industrial & Engineering Chemistry Research, 58(41):19292–19301. https://doi.org/10.1021/acs.iecr.9b04005
Yang SJ, Guo YF, Yan NQ, et al., 2011a. Capture of gaseous elemental mercury from flue gas using a magnetic and sulfur poisoning resistant sorbent Mn/γ-Fe2O3 at lower temperatures. Journal of Hazardous Materials, 186(1):508–515. https://doi.org/10.1016/j.jhazmat.2010.11.034
Yang SJ, Guo YF, Yan NQ, et al., 2011b. Elemental mercury capture from flue gas by magnetic Mn−Fe spinel: effect of chemical heterogeneity. Industrial & Engineering Chemistry Research, 50(16):9650–9656. https://doi.org/10.1021/ie2009873
Yang SJ, Guo YF, Yan NQ, et al., 2011c. Nanosized cation-deficient Fe−Ti spinel: a novel magnetic sorbent for elemental mercury capture from flue gas. ACS Applied Materials & Interfaces, 3(2):209–217. https://doi.org/10.1021/am100835c
Yang Y, Zhang SZ, Wang SW, et al., 2015. Ball milling synthesized MnOx as highly active catalyst for gaseous POPs removal: significance of mechanochemically induced oxygen vacancies. Environmental Science & Technology, 49(7): 4473–4480.
Ye D, Wang XX, Wang RX, et al., 2021. Recent advances in MnO2-based adsorbents for mercury removal from coal-fired flue gas. Journal of Environmental Chemical Engineering, 9(5):105993. https://doi.org/10.1016/j.jece.2021.105993
Ye D, Wang RX, Wang XX, et al., 2022a. Effects of synthesis methods on the physicochemical properties and Hg0 capture capability of MnO2−CeO2 mixed oxides. Applied Surface Science, 578:151998. https://doi.org/10.1016/j.apsusc.2021.151998
Ye D, Wang RX, Wang XX, et al., 2022b. Improvement in the Hg0 removal performance of CeO2 by modifying with CuO. Applied Surface Science, 579:152200. https://doi.org/10.1016/j.apsusc.2021.152200
Ye D, Wang XX, Wang RX, et al., 2022c. Relationship between Hg0 capture performance and physicochemical properties of CeO2−CrOx mixed oxides. Journal of Environmental Chemical Engineering, 10(5):108252. https://doi.org/10.1016/j.jece.2022.108252
Ye D, Wang XX, Wang RX, et al., 2022d. Review of elemental mercury (Hg0) removal by CuO-based materials. Journal of Zhejiang University-SCIENCE A (Applied Physics & Engineering), 23(7):505–526. https://doi.org/10.1631/jzus.A2100627
Zhao HT, Ezeh CI, Yin SF, et al., 2020. MoO3-adjusted δ-MnO2 nanosheet for catalytic oxidation of Hg0 to Hg2+. Applied Catalysis B: Environmental, 263:117829. https://doi.org/10.1016/j.apcatb.2019.117829
Acknowledgments
This work is supported by the Fundamental Research Funds of China Jiliang University and the Zhejiang Provincial Natural Science Foundation of China (No. LQ22E060003).
Author information
Authors and Affiliations
Contributions
Yongjin HU and Zhichang JIANG designed the research. Dong YE processed the corresponding data. Dong YE wrote the first draft of the manuscript. Xin LIU helped to organize the manuscript. Haining WANG and Dong YE revised and edited the final version.
Corresponding authors
Additional information
Conflict of interest
Dong YE, Yongjin HU, Zhichang JIANG, Xin LIU, and Haining WANG declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Ye, D., Hu, Y., Jiang, Z. et al. Mechanistic investigation on Hg0 capture over MnOx adsorbents: effects of the synthesis methods. J. Zhejiang Univ. Sci. A 24, 80–90 (2023). https://doi.org/10.1631/jzus.A2200388
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1631/jzus.A2200388