Abstract
This study aimed to establish a closed-cycle operation technology with high thermal efficiency in the thermochemical sulfur-iodine cycle for large-scale hydrogen production. A series of experimental studies were performed to investigate the occurrence of side reactions in both the H2SO4 and HI x phases from the H2SO4/HI/I2/H2O quaternary system within a constant temperature range of 323–363 K. The effects of iodine content, water content and reaction temperature on the side reactions were evaluated. The results showed that an increase in the reaction temperature promoted the side reactions. However, they were prevented as the iodine or water content increased. The occurrence of side reactions was faster in kinetics and more intense in the H2SO4 phase than in the HI x phase. The sulfur or hydrogen sulfide formation reaction or the reverse Bunsen reaction was validated under certain conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Hydrogen is one of the most promising energy vectors for the future because of its renewable and versatile characteristics. The production of hydrogen from water rather than from fossil fuels offers the potential to build a carbon free energy system. Among the proposed innovative approaches for mass hydrogen production, the thermochemical sulfur-iodine (SI or IS) cycle has attracted much interest in terms of its efficiency and cost (Brown et al., 2000; Goldstein et al., 2005). The cycle involves decomposing water molecules into hydrogen and oxygen via the three following steps, driven by thermal energy from an external source, according to a reference process scheme proposed by general atomics (Norman et al., 1982).
Fig. 1 shows that the Bunsen reaction Eq. (1) consists of the reaction between sulfur dioxide, iodine, and water to generate hydriodic and sulfuric acids. The resulting acid solution spontaneously splits into two nonmiscible liquid phases in the presence of excess water and iodine (Norman et al., 1982). The lighter phase (H2SO4 phase) contains predominantly H2SO4 and the heavier phase (HI x phase) mainly iodine in aqueous hydriodic acid (O’Keefe et al., 1982). Both phases are individually introduced into the H2SO4 and HI decomposition reactions after the purification and concentration steps at each decomposition section. This summary three-step reaction scheme regenerates the reagents, SO2 and I2, and splits water into hydrogen and oxygen.
Non-negligible amounts of sulfur-containing and iodine-containing compounds are dissolved in the HI x phase and H2SO4 phase, respectively (Giaconia et al., 2007). The reverse Bunsen reaction or side reaction between HI and H2SO4 may occur, producing SO2, elemental sulfur or H2S (Sakurai et al., 2000a; Hwang et al., 2005):
A number of problems need to be solved to optimize and industrialize the SI process, including the establishment of a closed-cycle operation technology, improvement of the process thermal efficiency, and development of the process unit materials (Sakurai et al., 2000a). Establishing the closed-cycle operation technology and improving the process thermal efficiency are both highly sensitive to several factors involved in the Bunsen section, such as the composition of the process solution, the excess of iodine and water in the feed, and the side reactions. The study of the Bunsen section has attracted most interest, and the majority of studies have focused on the performance of the liquid-liquid equilibrium phase separation (Sakurai et al., 2000b; Colette et al., 2006; Lee et al., 2006; Giaconia et al., 2007; Lee et al., 2008; Yoon et al., 2009; Colette-Maatouk et al., 2009; Zhu et al., 2012). The iodine content has been found to be the key parameter that affects the separation characteristics: the higher the iodine content, the better is the segregation behavior. In addition, Parisi et al. (2011) attempted to determine the effect of the reaction temperature on the efficiency of the SO2 conversion into sulfuric acid. An increase in the reaction temperature lowers both the quantity of the absorbed SO2 and its conversion. However, only a few studies have been conducted on the occurrence of the side reaction in both aqueous acid phases that exist in the liquid phase separator (Sakurai et al., 2000a; Hwang et al., 2005). In the current study, the effects of excess iodine, excess water, and reaction temperature on the occurrence of the side reaction in both liquid phases were investigated. The study aimed to provide useful experimental data on the conditions under which the side reaction occurs to help establish a closed-cycle operation technology with high process thermal efficiency.
2 Experimental procedures
2.1 Reagents and apparatus
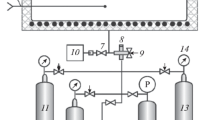
All chemicals used were of reagent grade. Deionized water was used throughout the procedure. Sulfuric acid (about 98%, in weight, Quzhou Juhua Reagent Co. Ltd., China) and hydriodic acid (about 56%, in weight, Shanghai Kefeng Industrial Co. Ltd., China) were systematically analyzed before use. The proton concentration in each acid was measured via acid-base titration. Each solution mixture of H2SO4/HI/I2/H2O was prepared in a 500 ml double-jacketed stirred reactor, where the temperature was controlled by a thermostatic water bath (Fig. 2). A SO2 and H2S gas analyzer (MOT400, Shenzhen Keernuo Technology Co. Ltd., China) connected to the reactor outlet qualitatively detected the by-products of the side reactions. Firstly, calculated amounts of aqueous hydriodic acid and iodine (>99.9%, Chinasun Specialty Products Co. Ltd., China) were introduced and evenly mixed to allow complete iodine dissolution. Subsequently, the mixture of water and sulfuric acid was added into the reactor. The solution mixture was then continuously stirred with a magnetic stirrer for 5 min.
2.2 Analysis and measurement
In the qualitative analysis, the formation of sulfur was confirmed by observation, and the formation of sulfur dioxide or hydrogen sulfide was proven using the SO2 and H2S gas analyzer. Some studies have shown that the side reactions that may occur between HI and H2SO4 have slow kinetics (Sakurai et al., 2000). Thus, the amounts of HI, H2SO4, and I2 in both HI x and H2SO4 phases after 10, 30, 60, and 90 min from the start of the reaction were measured using the chemical titration method.
The concentration of H+ was measured by sodium hydroxide (NaOH, Sinopharm Chemical Reagent Co. Ltd., China) titration after dilution of the sample in water. I– concentration was obtained by redox titration with 0.05 N potassium iodate (KIO3, Zhejiang Hichi Chemical Co. Ltd., China) standard solution after the sample was diluted in water. I2 concentration was determined by redox titration with 0.1 N sodium thiosulfate (Na2S2O3, Xiaoshan Chemical Reagent Co. Ltd., China) solution after dilution of the sample in a potassium iodide (KI, Aladdin Reagent (Shanghai) Co. Ltd., China) aqueous solution, preventing iodine from precipitating prior to the measurement. Assuming only four species (H2SO4, HI, I2, and H2O) constituted each phase, the concentrations of H2O and H2SO4 were calculated according to the mass and ionic balance. Several repeated samples were taken to assess the experimental accuracy and reliability in each operating condition. The relative experimental error was about ±5%, caused mainly by the measurement and analysis of the composition of the two liquid phases.
3 Results and discussion
A series of experiments were performed to study the influence of the amounts of iodine and water, and the reaction temperature on the Bunsen side reactions in both H2SO4 and HI x phases produced from the Bunsen section. The selected experimental parameters were based on the optimal operating range for the Bunsen process (Lee et al., 2008; Zhu et al., 2012).
3.1 Effect of iodine content on the Bunsen side reaction
The variations in the composition of the two liquid phases obtained with reaction times at 333, 343, 353, and 363 K are shown in Figs. 3–6, respectively, where the vertical axis represents the ratio between the amount of a component in the solution at time t and 10 min.
Results for the concentration ratio are shown in Fig. 3, where the molar ratio of H2SO4/HI/I2/H2O= 1/2/1.6/12 or 1/2/2.5/12, and in Figs. 4–6, where the molar ratio=1/2/2.5/12 or 1/2/3.5/12. The amounts of H2SO4 and HI in both liquid phases decreased with the reaction time, indicating that side reactions between H2SO4 and HI were occurring. The increase in the amount of iodine in the medium resulted in a decrease in the change in the amounts of H2SO4 and HI in the H2SO4 phase within the temperature range of 333–363 K. This finding confirmed that the higher iodine content in the H2SO4 phase prevented occurrence of the side reactions. Side reactions in the HI x were effectively controlled as the iodine content increased in the temperature range of 333–363 K. Note that the side reactions had a different kinetic rate in the two liquid phases, which may be explained by different compositions of the H2SO4 and HI x phases. The higher quantity of iodine available in the HI x phase may have impeded the occurrence of Bunsen side reactions or reverse Bunsen reaction. The side reactions proceeded faster in the H2SO4 phase than in the HI x phase. Both H2S and sulfur formation side reactions Eqs. (5) and (6) respectively, were validated through qualitative analysis. This result was in agreement with the findings from previous studies (Sakurai et al., 2000a; Hwang et al., 2005).
3.2 Effect of water content on the Bunsen side reaction
The effect of excess water on Bunsen side reactions was analyzed at 323 K, with molar ratios of H2SO4/HI/I2/H2O=1/2/1.6/12 and 1/2/1.6/16. Variations in the composition in the H2SO4 and HI x phases are plotted in Figs. 7a and 7b, respectively. The amounts of H2SO4 and HI in both liquid phases decreased with the reaction time; the rate of decrease was evidently reduced as the excess water content increased. Therefore, an increase in water content prevented the side reactions. In these cases, the occurrence of both the sulfur formation reaction Eq. (5) and the reverse Bunsen reaction Eq. (4) was detected by the SO2 and H2S gas analyzer. The reaction products, H2O and I2, were easily transferred into the H2SO4 and HI x phases, respectively. An increase in water content and a decrease in I2 content were observed in the H2SO4 phase, whereas the opposite result was observed in the HI x phase. Therefore, the reaction products, water and I2, were easily transferred into the H2SO4 and HI x phases, respectively. This is because the existence of the strong complexation reaction between iodine and iodide and the higher amount of I2 in the HI x phase may expel H2O from the HI solution. The side reactions proceeded much faster and more intensely in the H2SO4 phase than in the HI x phase.
3.3 Effect of reaction temperature on the Bunsen side reaction
A close relationship exists between the liquid-liquid equilibrium phase separation and the reaction temperature during the Bunsen reaction. A number of tests were performed to investigate the influence of reaction temperature with molar ratios of H2SO4/HI/I2/H2O=1/2/1.6/12, 1/2/2.5/12 or 1/2/3.5/12 (Figs. 8–10) to explore further the occurrence of side reactions in the two liquid phases.
Figs. 8a and 8b show that the trends in the amounts of H2SO4 and HI in the H2SO4 and HI x phases were nearly coincident when the reaction temperature varied from 323 K to 333 K. The side reactions were badly affected by the reaction temperature. An increasing temperature, from 333 K to 363 K and from 343 K to 363 K, increased the reduction in the amounts of H2SO4 and HI in the H2SO4 and HI x phases when the molar ratio of H2SO4/HI /I2/H2O was 1/2/2.5/12 or 1/2/3.5/12 (Figs. 9 and 10). This result indicates that the side reactions were promoted as the reaction temperature increased. This phenomenon may be explained by the endothermic property of the Bunsen side reaction and the active kinetics of the composition in both acid phases at the higher reaction temperature.
The side reactions proceeded more intensively in the H2SO4 phase than in the HI x phase, possibly due to the large quantity of iodine, the product of the side reactions and the reverse Bunsen reaction, dissolved in the HI x phase.
4 Conclusions
Different experiments were performed to investigate the effects of excess iodine and water, and reaction temperature on the conditions for the occurrence of the Bunsen side reaction of the quaternary system H2SO4/HI/I2/H2O in the SI process. Variations in the composition of the two liquid phases with the reaction time were fully characterized using previously validated analytical methods. The formation of sulfur, hydrogen sulfide, and sulfur dioxide were validated by qualitative analysis. The occurrence of the side reaction was faster and more intense in the H2SO4 phase than in the HI x phase. Under certain conditions of the liquid-liquid equilibrium phase separation, sulfur formation reaction and reverse Bunsen reaction occurred at a reaction temperature of 323 K. H2S and sulfur formation side reactions proceeded in the temperature range of 333–363 K. In addition, an increase in the reaction temperature promoted side-reactions in the two liquid phases. Side reactions were prevented in both liquid phases when the amount of excess iodine or water increased.
References
Brown, L.C., Funk, J.F., Showalter, S.K., 2000. Initial Screening of Thermochemical Water-Splitting Cycles for High Efficiency Generation of Hydrogen Fuels using Nuclear Power. GA Project 30047.
Colette-Maatouk, S., Brijou-Mokrani, N., Carles, P., Fauvet, P., Tabarant, M., Dutruc-Rosset, C., Fleche, J.L., Borgard, J.M., 2006. Experimental Study of Bunsen Reaction in the Framework of Massive Hydrogen Production by the Sulfur-Iodine Thermochemical Cycle. Proceedings of WHEC, Lyon, France, p.13–16.
Colette-Maatouk, S., Brijou-Mokrani, N., Tabarant, M., Fleche, J.L., Carles, P., 2009. Study of the miscibility gap in H2SO4/HI/I2/H2O mixtures produced by the Bunsen reaction-Part I: Preliminary results at 308 K. International Journal of Hydrogen Energy, 34(17):7155–7161. [doi:10.1016/j.ijhydene.2009.06.033]
Giaconia, A., Caputo, G., Ceroli, A., Diamanti, M., Barbarossa, V., Tarquini, P., Sau, S., 2007. Experimental study of two phase separation in the Bunsen section of the sulfur-iodine thermochemical cycle. International Journal of Hydrogen Energy, 32(5):531–536. [doi:10.1016/j.ihydene.2006.08.015]
Goldstein, S., Borgard, J.M., Vitart, X., 2005. Upper bound and best estimate of the efficiency of the iodine sulphur cycle. International Journal of Hydrogen Energy, 30(6): 619–626. [doi:10.1016/j.ijhydene.2004.06.005]
Hwang, G.J., Kim, Y.H., Park, C.S., Lee, S.H., Kim, C.H., Bae, K.K., 2005. Bunsen Reaction in IS (Iodine-Sulfur) Process for the Thermochemical Hydrogen Production. Proceedings of International Hydrogen Energy Congress and Exhibition (IHEC), Istanbul, Turkey, p.13–15.
Lee, B.J., No, H.C., Yoon, H.J., Kim, S.J., Kim, E.S., 2008. An optimal operating window for the Bunsen process in the I-S thermochemical cycle. International Journal of Hydrogen Energy, 33(9):2200–2210. [doi:10.1016/j.ijhydene.2008.02.045]
Lee, D.H., Lee, K.J., Kang, Y.H., Kim, Y.H., Park, C.S., Hwang, G.J., 2006. High temperature phase separation of H2SO4-HI-H2O-I2 system in iodine-sulfur hydrogen production process. Transactions of the Korean Hydrogen and New Energy Society, 17(4):395–402.
Norman, J., Besenbruch, G., Brown, L., O’Keefe, D., Allen, C., 1982. Thermochemical Water-Splitting Cycle, Bench-Scale Investigations, and Process Engineering, Final Report for the Period February 1977 through December 31, 1981. General Atomics Report GA-A16713, DOE Report DOE/ET/26225-1.
O’Keefe, D., Allen, C., Besenbruch, G., Brown, L., Norman, J., Sharp, R., McCorkle, K., 1982. Preliminary results from bench-scale testing of a sulfur-iodine thermochemical water-splitting cycle. International Journal of Hydrogen Energy, 7(5):381–392. [doi:10.1016/0360-3199(82)90048-9]
Parisi, M., Giaconia, A., Sau, S., Spadoni, A., Caputo, G., Tarquini, P., 2011. Bunsen reaction and hydriodic phase purification in the sulfur-iodine process: An experimental investigation. International Journal of Hydrogen Energy, 36(3):2007–2013. [doi:10.1016/j.ijhydene.2010.11.039]
Sakurai, M., Nakajima, H., Amir, R., Onuki, K., Shimizu, S., 2000a. Experimental study on side-reaction occurrence condition in the iodine-sulfur thermochemical hydrogen production process. International Journal of Hydrogen Energy, 25(7):613–619. [doi:10.1016/S0360-3199(99)00074-9]
Sakurai, M., Nakajima, H., Onuki, K., Shimizu, S., 2000b. Investigation of 2 liquid phase separation characteristics on the iodine-sulfur thermochemical hydrogen production process. International Journal of Hydrogen Energy, 25(7):605–611. [doi:10.1016/S0360-3199(99)00078-6]
Yoon, H.J., No, H.C., Kim, Y.S., Jin, H.G., Lee, J.I., Lee, B.J., 2009. Demonstration of the I-S thermochemical cycle feasibility by experimentally validating the over-azeotropic condition in the hydroiodic acid phase of the Bunsen process. International Journal of Hydrogen Energy, 34(19):7939–7948. [doi:10.1016/j.ijhydene.2009.07.100]
Zhu, Q.Q., Zhang, Y.W., Zhou, C., Wang, Z.H., Zhou, J.H., Cen, K.F., 2012. Optimization of liquid-liquid phase separation characteristics in the Bunsen section of the sulfur-iodine hydrogen production process. International Journal of Hydrogen Energy, 37(8):6407–6414. [doi:10.1016/j.ijhydene.2012.01.044]
Author information
Authors and Affiliations
Corresponding author
Additional information
Project (No. 51006088) supported by the National Natural Science Foundation of China
Rights and permissions
About this article
Cite this article
Zhu, Qq., Zhang, Yw., Ying, Z. et al. Occurrence of the Bunsen side reaction in the sulfur-iodine thermochemical cycle for hydrogen production. J. Zhejiang Univ. Sci. A 14, 300–306 (2013). https://doi.org/10.1631/jzus.A1200313
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1631/jzus.A1200313