Abstract
Objective
To study the stereoselective glucuronidation of carvedilol (CARV) by three Chinese liver microsomes.
Methods
The metabolites of CARV were identified by a hydrolysis reaction with β-glucuronidase and HPLC-MS/MS. The enzyme kinetics for CARV enantiomers glucuronidation was determined by a reversed phase-high pressure liquid chromatography (RP-HPLC) assay using (S)-propafenone as internal standard after precolumn derivatization with 2,3,4,6-tetra-O-acetyl-β-D-glucopyranosylisothiocyanate.
Results
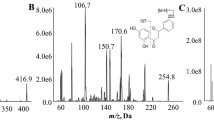
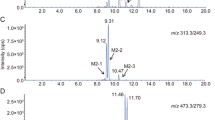
Two CARV glucuronides were found in three Chinese liver microsomes incubated with CARV. The non-linear regression analysis showed that the values of K m and V max for (S)-CARV and (R)-CARV enantiomers were (118±44) μmol/L, (2500±833) pmol/(min·mg protein) and (24±7) μmol/L, (953±399) pmol/(min·mg protein), respectively.
Conclusion
These results suggested that there was a significant (P<0.05) stereoselective glucuronidation of CARV enantiomers in three Chinese liver microsomes, which might partly explain the enantioselective pharmacokinetics of CARV.
Similar content being viewed by others
References
Eggertsen, R., Sivertsson, R., Andren, L., Hansson, L., 1987. Acute and long-term hemodynamic effects of carvedilol, a combined beta-adrenoceptor blocking and precapillary vasodilating agent, in hypertensive patients. J. Cardiovasc. Pharmacol., 10(Suppl. 11):97–100.
Fujimaki, M., 1994. Oxidation of R(+)-and S(−)-carvedilol by rat liver microsomes. Evidence for stereoselective oxidation and characterization of the cytochrome P450 isozymes involved. Drug Metab. Dispos., 22(5):700–708.
Fujimaki, M., Murakoshi, Y., Hakusui, H., 1990. Assay and disposition of carvedilol enantiomers in humans and monkeys: evidence of stereoselective presystemic metabolism. J. Pharm. Sci., 79(7):568–572. [doi:10.1002/jps.2600790704]
Fujimaki, M., Shintani, S., Hakusui, H., 1991. Stereoselective metabolism of carvedilol in the rat. Use of enantiomerically radiolabeled pseudoracemates. Drug Metab. Dispos., 19(4):749–753.
Gibbson, G.G., Shett, P., 1994. Introduction to Drug Metabolism, 2nd Ed. Blackie Academic and Professional, London, p.217–221.
Green, M.D., Tephly, T.R., 1996. Glucuronidation of amines and hydroxylated xenobiotics and endobiotics catalyzed by expressed human UGT1.4 protein. Drug Metab. Dispos., 24(3):356–363.
Honda, M., Nozawa, T., Igarashi, N., Inoue, H., Arakawa, R., Ogura, Y., Okabe, H., Taquchi, M., Hashimoto, Y., 2005. Effect of CYP2D6*10 on the pharmacokinetics of R-and S-carvedilol in healthy Japanese volunteers. Biol. Pharm. Bull., 28(8):1476–1479. [doi:10.1248/bpb.28.1476]
Lowry, O.H., Rosebrough, N.J., Farr, A.L., Randall, R.J., 1951. Protein measurement with the Folin phenol reagent. J. Biol. Chem., 193(1):265–275.
Morgan, T., 1994. Clinical pharmacokinetics and pharmacodynamics of carvedilol. Clin. Pharmacokinet., 26(5):335–346.
Nägele, H., Bohlmann, M., Eck, U., Petersen, B., Rodiger, W., 2000. Combination therapy with carvedilol and amiodarone in patients with severe heart failure. Eur. J. Heart Fail., 2(1):71–79. [doi:10.1016/S1388-9842(99)00071-9]
Nahrendorf, W., Rading, A., Steinig, G., van der Does, R., Schlote, A., 1992. A comparison of carvedilol with a combination of propranolol and isosorbide dinitrate in the chronic treatment of stable angina. J. Cardiovasc. Pharmacol., 19(Suppl. 1):114–116.
Neugebauer, G., Neubert, P., 1991. Metabolism of carvedilol in man. Eur. J. Drug Metab. Pharmacokinet., 16(4):257–260.
Neugebauer, G., Akpan, W., von Mollendorff, E., Neubert, P., Reiff, K., 1987. Pharmacokinetics and disposition of carvedilol in humans. J. Cardiovasc. Pharmacol., 10(Suppl. 11):85–88.
Neugebauer, G., Akpan, W., Kaufmann, B., Reiff, K., 1990. Stereoselective disposition of carvedilol in man after intravenous and oral administration of the racemic compound. Eur. J. Clin. Pharmacol., 38(Suppl. 2):108–111. [doi:10.1007/BF01409476]
Ohno, A., Saito, Y., Hanioka, N., Jinno, H., Saeki, M., Ando, M., Ozawa, S., Sawada, J., 2004. Involvement of human hepatic UGT1A1, UGT2B4, and UGT2B7 in the glucuronidation of carvedilol. Drug Metab. Dispos., 32(2):235–239. [doi:10.1124/dmd.32.2.235]
Oldham, H.G., Clarke, S.E., 1997. In vitro identification of the human cytochrome P450 enzymes involved in the metabolism of R(+)-and S(−)-carvedilol. Drug Metab. Dispos., 25(8):970–977.
Packer, M., Bristow, M.R., Cohn, J.N., Colucci, W.S., Fowler, M.B., Gilbert, E.M., Shusterman, N.H., 1996. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. N. Engl. J. Med., 334(21):1349–1355. [doi:10.1056/NEJM199605233342101]
Radominska-Pandya, A., Czernik, P.J., Little, J.M., Battaglia, E., Mackenzie, P.I., 1999. Structural and functional studies of UDP-glucuronosyltransferases. Drug Metab. Rev., 31(4):817–899. [doi:10.1081/DMR-100101944]
Ruffolo, R.R.Jr, Boyle, D.A., Venuti, R.P., Lukas, M.A., 1993. Preclinical and clinical pharmacology of carvedilol. J. Hum. Hypertens., 7(Suppl. 1):2–15.
Saito, M., Kawana, J., Ohno, T., Kaneko, M., Mihara, K., Hanada, K., Suqita, R., Okada, N., Oosato, S., Naqayama, M., Sumiyoshi, T., Oqata, H., 2006. Enantioselective and highly sensitive determination of carvedilol in human plasma and whole blood after administration of the racemate using normal-phase high-performance liquid chromatography. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci., 843(1):73–77. [doi:10.1016/j.jchromb.2006.05.018]
Schaefer, W.H., 1992. Formation of a carbamoyl glucuronide conjugate of carvedilol in vitro using dog and rat liver microsomes. Drug Metab. Dispos., 20(1):130–133.
Sorich, M.J., McKinnon, R.A., Miners, J.O., Smith, P.A., 2006. The importance of local chemical structure for chemical metabolism by human uridine 5-diphosphateglucuronosyltransferase. J. Chem. Inf. Model., 46(6):2692–2697. [doi:10.1021/ci600248e]
Sponer, G., Muller-Beckmann, B., 1983. Studies on the mechanisms on the vasodilating activity of BM 14190. Naungy-Schmiedeberg’s Archies of Pharmacology, 322(Suppl.):R46.
Stahl, E., Henke, D., Mutschler, E., Spahn-Langguth, H., 1993. Saturable enantioselective first-pass effect for carvedilol after high oral racemate doses in rats. Archiv Der Pharmazie, 326(3):123–125. [doi:10.1002/ardp.19933260302]
Takekuma, Y., Takenaka, T., Kiyokawa, M., Yamazaki, K., Okamoto, H., Kitabatake, A., Tsutsui, H., Suqawara, M., 2006. Contribution of polymorphisms in UDP-glucuronosyltransferase and CYP2D6 to the individual variation in disposition of carvedilol. J. Pharm. Pharm. Sci., 9(1):101–112.
Takekuma, Y., Takenaka, T., Kiyokawa, M., Yamazaki, K., Okamoto, H., Kitabatake, A., Tsutsui, H., Suqawara, M., 2007. Evaluation of effects of polymorphism for metabolic enzymes on pharmacokinetics of carvedilol by population pharmacokinetic analysis. Biol. Pharm. Bull., 30(3):537–542. [doi:10.1248/bpb.30.537]
van Zwieten, P.A., 1993. Pharmacodynamic profile of carvedilol. Cardiology, 82(Suppl. 3):19–23.
Xie, S.G., Chen, Y.K., Chen, S.Q., Zeng, S., 2006. Glucuronidation of apigenin by the recombinant human UGT1A3. Chin. J. Pharmacol. Toxicol., 20(5):405–409 (in Chinese).
Yang, E., Wang, S., Kratz, J., Cyronak, M.J., 2004. Stereoselective analysis of carvedilol in human plasma using HPLC/MS/MS after chiral derivatization. J. Pharm. Biomed. Anal., 36(3):609–615. [doi:10.1016/j.jpba.2004.07.008]
Yao, T.W., Zeng, S., 2001. Stereoselective determination of phydroxyphenyl-phenylhydantoin enantiomers in rat liver microsomal incubates by reversed-phase high-performance liquid chromatography using β-cyclodextrin as chiral mobile phase additives. Biomed. Chromatogr., 15(2):141–144. [doi:10.1002/bmc.50]
Yao, T.W., Zhou, Q., Zeng, S., 2000. Stereoselective determination of propafenone enantiomers in transgenic Chinese hamster CHL cells expressing human cytochrome P450. Biomed. Chromatogr., 14(7):498–501. [doi:10.1002/1099-0801(200011)14:7〈498::AID-BMC9〉3.0.CO;2-D]
Zeng, S., Zhong, J., Pan, L., Li, Y., 1999. HPLC separation and quantitation of ofloxacin enantiomes in rat microsomes. J. Chromatogr. B Biomed. Sci. Appl., 728(1):151–155. [doi:10.1016/S0378-4347(99)00085-7]
Zhou, H.H., Wood, A.J., 1995. Stereoselective disposition of carvedilol is determined by CYP2D6. Clin. Pharmacol. Ther., 57(5):518–524. [doi:10.1016/0009-9236(95)90036-5]
Author information
Authors and Affiliations
Corresponding author
Additional information
Project supported by the National Natural Science Foundation of China (No. 30225047) and the Science and Technology Foundation of Zhejiang Province, China (No. 2005C13026)
Rights and permissions
About this article
Cite this article
You, Ly., Yu, Cn., Xie, Sg. et al. Stereoselective glucuronidation of carvedilol by Chinese liver microsomes. J. Zhejiang Univ. - Sci. B 8, 756–764 (2007). https://doi.org/10.1631/jzus.2007.B0756
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1631/jzus.2007.B0756