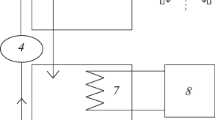
Abstract
Study on the effects of sonolysis, ozonolysis and US/O3 system on the decomposition of p-chlorophenol in aqueous solutions indicated that in the cases of US/O3 system, individual ozonolysis and sonolysis, the decomposition rate of p-chlorophenol reached 78/78%, 56.20%, 2.79% after a 16-min reaction while its CODcr (chemical oxygen demand) removal rate was 97.02%, 62.17%, 3.67% after a 120-min reaction. The decomposition reaction of p-chlorophenol follows pseudo-first-order kinetics. The enhancement factors of p-chlorophenol and its CODcr under US/O3 system reached 63% and 237% respectively. The main intermediates during the decomposition include catechol, hydroquinone, p-benzoquinone, phenol, fumaric acid, maleic acid, oxalic acid and formic acid. The decomposition mechanism of p-chlorophenol was also discussed.
Similar content being viewed by others
References
Adams, C.D., Scanlan, P.A., Secrist, N.S., 1994. Oxidation and biodegradability enhancement of 1,4-dioxane using hydrogen peroxide and ozone.Environ. Sci. Technol.,28:1812–1818.
Bader, H., Hoigné, J., 1981. Determination of ozone in water by the indigo method.Wat. Res.,15:449–456.
Dahi, E., 1976. Physicohemical aspects of disinfection of water by means of ultrasound and ozone.Wat. Res.,10:677–684.
Destaillats, H., Colussi, A.J., Joseph, J.M., Hoffmann, M.R., 2000. Synergistic effects of sonolysis combined with ozonolysis for the oxidation of azobenzene and methyl orange.J. Phys. Chem. A.,104:8930–8935.
EPA (Environment Protection Bureau), 1989. Standard Methods for the Examination of Water and Wastewater. American Public Health Association, Washington DC, p. 4–162.
Guittoneau, S., Duguet, J.P., Bonnel, C., Dore, M., 1990. Oxidation of parachloronitrobenzene in dilute aqueous solution by O3+UV and H2O2+UV: A comparative study.Ozone. Sci. Eng.,12:73–94.
Hart, E.J., Henglein, A., 1986. Sonolysis of ozone in aqueous solution.J. Phys. Chem.,90:3061–3062.
Kang, J.W., Hoffmann, M.R., 1998. Kinetics and mechanism of the sonolytic destruction of methyl tert-butyl ether by ultrasonic irradiation in the presence of ozone.Environ. Sci. Technol.,32:3194–3199.
Mokrini, A., Oussi, D., Esplugas, S., 1997. Oxidation of aromatic compounds with UV radiation/ozone/hydrogen peroxide.Water. Sci. Technol.,35(4):95–102.
Olson, T.M., Baraier, P.F., 1994. Oxidation kinetics of natural organic matter by sonolysis and ozone.Wat. Res.,28(6):1383–1391.
Peyton, G.R., Glaze, W.H., 1988. Destruction of pollutants in water with oxone in combination with ultraviolet radiation. 3. Photolysis of aqueous ozone.Environ. Sci. Technol.,22:761–767.
Sierka, R.A., Amy, G.L., 1985. Catalytic effects of ultraviolet light and/or ultrasound on the ozone oxidation of humic acid and trihalomethane precursors.Ozone. Sci. Eng., 7:47–62.
Staehelin, J., Hoigné, J., 1982. Decomposition of ozone in water: Rate of initiation by hydroxide ions and hydrogen peroxide.Environ Sci Technol,16:676–681.
Xi, D.L., Shun, Y.S., Liu, X.Y., 1989. Environmental Mensuration. China Publishing Company of Higher Education, Beijing (in Chinese).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xu, Xw., Xu, Xh., Shi, Hx. et al. Study on US/O3 mechanism in p-chlorophenol decomposition. J Zheijang Univ Sci B 6, 553–558 (2005). https://doi.org/10.1631/jzus.2005.B0553
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1631/jzus.2005.B0553