Abstract
The application of carbon fiber (CF) reinforced cementitious composites is often restricted by the poor load transfer between the components. In this study, two reactive coating materials—nano-silica and micro-silica—are utilized to modify the CF surfaces via an electrophoretic deposition approach. These negatively charged particles are able to be electrosorbed onto the fiber surfaces under a constant electrical field according to the zeta and cyclic voltammetry measurements. After surface treatment, XRD analysis of CFs showed an increase in graphite crystallite thickness and decrement in interlayer spacing \({d}_{002}\), significantly affecting the fiber diameters. Additionally, the lengths of crystallites were reduced, which can impair the fiber strength and their temperature stability. Single fiber pullout tests exhibited that the interfacial bonding can be clearly enhanced by both reactive coatings. Microscopic observation revealed that C–S–H gel and calcite structures can be formed near the fiber surfaces after immersion in cement pore solution owing to pozzolanic reaction and nucleation effect, tremendously heightening both chemical and mechanical interaction between fiber and cement. Finally, based on a detailed micromechanical analysis, the reinforcing mechanisms between the differently modified fibers and the cementitious matrix were elaborated and discussed.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Cement and concrete composites are crucial materials in the construction of modern society, since they are extensively used in numerous engineered structures such as bridges, roads, ports, dams, tunnels, and large and high-rise residential buildings [1,2,3]. In 2019, the worldwide production of cement is estimated to reach nearly 4.1 Gt [4, 5], making it the second most widely used material on earth after water [6]. Nevertheless, cement-based materials have several limitations such as poor tensile/flexural strength, low fracture toughness, and crack-proneness, which confines their free-form design and serviceability [7]. To strengthen its mechanical properties, carbon fiber (CF) has garnered significant attention in recent years as a reinforcement material, owing to its excellent tensile properties and considerably low density [8, 9]. Meanwhile, as opposed to ordinary steel reinforcement, CFs have superior chemical resistance; therefore, no protective concrete cover is required, enabling the thin-walled, lightweight, and sophisticated, structural members [10].
However, CFs with chemically inert and hydrophobic surfaces have weak bonding toward cement-based or other mineral-based composites, which cannot ensure the stress transfer between two components and the full utilization of fibers’ high-strength properties [11, 12]. This issue strongly restricts the development and application of CF-reinforced cementitious composites (CFRC) in the construction industry. To this end, in a previous publication [7], the authors have performed a surface modification of CFs by anodic oxidation in the cementitious pore solution; and successfully coated dense, nanostructured calcite layers onto fiber surfaces. Their outcome suggested a drastic enhancement in the bond performance of CF to the cement matrices after electrochemical treatment. However, higher voltages and longer treatment duration had a negative effect on the physical properties of fibers.
Electrophoretic deposition (EPD) is also a promising material processing technique, which has been frequently used to decorate CF surfaces [13]. During EPD treatment, the charged particles suspended in the liquid could move toward the electrode surfaces with opposite charges under an electric field, forming homogeneous films or coatings [14]. The nature of good electrical conductivity and chemical stability for CF also allows the feasibility of this material to be used as an electrode during the EPD process [15].
So far, the EPD technique has mainly been utilized to modify the surfaces of CFs for an enhanced bond behavior with polymer matrices [9, 13]. For cement-based matrices, surface modification by applying EPD for CFs has aroused increasing interest in recent years [8, 16, 17]. Lu et al. [11] have coated the short CFs with graphene oxide (GO) layers by using EPD and the results demonstrated that the coatings with abundant functional groups provoked a better interfacial interaction between CF and cementitious matrices. In the studies of Li et al. [10, 12, 18], CFs were electrophoretically coated with negatively charged nano-silica (NS) colloids, bringing about the pronounced augmentation of bond strength of CF to cement at various curing ages. They revealed that the deposited NS particles facilitated the formation of calcite structures and calcium silicate hydrate (C–S–H) gel onto fiber surfaces due to their pozzolanic reactivity and nucleating effect. Micro-quartz particles have been also explored to modify the CF surfaces via EPD, achieving satisfied enhancement in interfacial bonding between fiber and cement matrix [17].
Silica fume (also often termed ‘micro-silica’) seems to be a superior choice as the deposit material for decorating CF surfaces in the EPD regime owing to its negative surface charge [19, 20]. This material, which is primarily composed of amorphous silicon dioxide (SO2), is a by-product in the manufacture of silicon and ferrosilicon alloy [21]. Due to its high pozzolanic reactivity and very small particle size, it has been commonly employed to accelerate hydration, refine the pore structures, and improve the mechanical performance as well as durability in the cement-based system [19, 21]. According to the literature, both NS and MS materials have been exploited to tailor the interfacial interaction between CF and cement-based matrices [22, 23]. For example, Nadiv et al. [22] have coated CF bundle by impregnation with MS, achieving a remarkable enhancement in bond behavior of multifilament toward cementitious composite; while the applied NS coatings with relatively large surface areas provoked inferior bond owing to their extensive agglomeration. However, the use of micro-silica (MS) for modifying the CFs through EPD treatment has not yet been reported so far.
In this study at hand, the CF surfaces were electrophoretically coated with nano-silica (NS) and micro-silica (MS) by applying the EPD approach so as to strengthen the bonding at the fiber-cement interface. Additionally, the CF bundles are stored in the cementitious pore solution for a deep insight into the reaction of coating materials in the cementitious environment. The CF properties are evaluated by means of thermogravimetric analysis, electron microscopy, and single-fiber tensile tests. Zeta potential analysis and cyclic voltammetry measurements are performed to explore the electrophoretic motion of particles and the deposition process. Furthermore, single-fiber pullout tests are carried out to compare the reinforcing effect in interfacial bonding among various coating materials, and the obtained bond-slip curves are comprehensively assessed to investigate the pullout mechanism.
2 Materials and methods
2.1 Materials
Commercially available Poly-acrylonitrile-based CF multifilaments sized with epoxy were bought from the SGL Group (SIGRAFIL® C T50-4.4/255-E100, Wiesbaden, Germany), whose main physical properties are given in Table S1. A type of submicron-sized silica fume (termed ‘micro-silica’; Elkem Microsilica® 971, Elkem, Norway; whose chemical composition can be seen in Table S2) and a porous, spherical nano-silica material (termed ‘nano-silica’; 637,246, Sigma-Aldrich, Munich, Germany, with a density of 2.2–2.6 g/cm3 and a particle size of 5–15 nm, according to the provider; whose trace metal concentrations can be seen in Table S3) were utilized for the CF surface modification. Their particle diameter distributions were measured by using a laser diffraction particle size analyzer (LS 13320, Beckman Coulter, Inc., Fullerton, USA) and listed in Fig. S1. Note that NS powder exhibited higher particle size and wider diameter distribution as compared to MS, which could be owed to the pronounced agglomeration of NS particles. Thus, the data of NS particle size distribution cannot be adopted in this study.
For each suspension used for the EPD process, 1 wt% NS, 1 wt% MS, and 1 wt% particle combinations of NS and MS (with a mass ratio of 1:1) were dispersed in the distilled water via ultrasonication treatment (at 70% amplitude for 5 min) using a Bandelin Sonopuls HD 2070 ultrasonicator (Bandelin Electronics, Berlin, Germany). The cement-based matrices used for single-fiber pullout measurements are adapted from previous investigations [7, 10, 12], whose composition is listed in Table S4.
2.2 Electrophoretic deposition
In the EPD system, the CF roving is used as an anode and a graphite cylinder acts as a cathode. Both electrodes are immersed in an aqueous suspension with 1 wt% silica particles. The electrical field in the regime was offered by a direct current (DC) power supply using a Voltcraft LPS 1305 device (Conrad Electronic AG, Wollerau, Switzerland). On the basis of previous research [10, 24], a parameter set of 1 V, 15 min was adopted to avoid the negative impact of water electrolysis and achieve a good deposition quality. After finishing the EPD modification, the treated CF yarns were taken out from the suspension and then cleaned with distilled water four times. Subsequently, the specimens were dried under normal laboratory conditions (with 55% relative humidity at 24 °C) for more than one day. Furthermore, the CFs modified with various silica combinations were stored in a cementitious pore solution for 28 days, with the aim to deliver deep insights into the reaction between modified CFs and cement-based matrices. To prepare the cementitious pore solution, 1.25 kg of CEM I 52.5 R HS/NA Portland cement purchased from Holcim AG, Germany, was first added to 6.5 L of tap water in a bucket. This mixture was then stirred for two days. Finally, the transparent liquid in the upper part was filtered using filter paper.
2.3 Experimental characterization
The morphological appearance of CF surfaces was characterized by environmental scanning electron microscopy (ESEM) by means of a Quanta 250 FEG instrument (FEI, The Netherlands). X-ray diffraction (XRD; 3003-TT, Seifert, Ahrensburg, Germany) analysis was performed to explore the crystalline structures of coating materials and graphite of CFs. The thermal stability of the surficial coatings and CF itself was investigated by using thermo-gravimetric analysis (TGA; NETZSCH Gerätebau GmbH, Selb, Germany) in a pure oxygen stream (with a heating speed of 10 °C/min and a flow rate of 60 ml/min). Zeta potential measurements were carried out using a Field ESA device (PA Partikel-Analytik-Messgeräte GmbH, Frechen, Germany) for dispersions at ambient temperature. To study the deposition process and electrode reaction kinetics, the cyclic voltammetry (CV) analysis was performed using an electrochemical workstation (CHI660E, USA) at a 9 mV/s scanning rate in the voltages ranging from 0 to 1 V.
To test the fiber strength, a Zwick Roell 1445 testing machine (Zwick Roell, Ulm, Germany) with a 10 N force cell was utilized to perform the single-fiber tension tests at a loading rate of 0.01 mm/s. 20 mm was selected as the free length of fibers under investigation. For each parameter, over 36 repeated samples were measured. Furthermore, the single-fiber pullout tests were conducted by using the Zwick Roell 1445 testing machine (with a 10 N load cell and a loading speed of 0.01 mm/s) to assess the bond quality of CF to cementitious matrices. To prepare the pullout samples, special molds with a 1.2 mm spacer were utilized to ensure a consistent embedded length of fibers [12]. The fresh mixture was poured into the molds and cured in sealed plastic bags for 2 days before demolding. Subsequently, all the samples were stored under constant laboratory conditions (24 °C, 55% RH) for 26 days until testing. At least 10 specimens were measured for every group. Details of the setup configuration for single-fiber tension tests and pullout tests were reported in the previous studies [7, 10, 12, 25].
3 Results and discussion
3.1 Zeta potential of the used silica suspensions
Table 1 presents the results of zeta potential ζ measurements for all dispersions. The zeta potentials for NS, MS, and NS/MS suspensions are −20 mV, −11 mV, and −13 mV, respectively, denoting an incipient instability of these negatively charged colloids in the aqueous solution, as referred to ASTM Standard (D 4187-82) [26].
The negative potentials of those dispersions can be traced back to the deprotonation of their silanol groups (–Si–OH) on the surfaces of silica gels [27]. These Si–OH groups are expected to be like a weak acid (K1 = 10–9.8) [27], causing the slightly acidic pH in the NS suspensions. However, MS suspension presented an alkaline pH of 9.42, since the silica fume originated from the industrial by-product containing some alkali metal oxides, such as K2O, and Na2O, which could strongly affect the pH environment and ionic concentration. As well, MS dispersion showed the lowest absolute value of ζ-potential among those samples, indicating the lowest stability. The pH of NS/MS suspension is almost neutral (pH = 7.02), exhibiting very low zeta values of about −13 mV. According to Depasse and Watillon [28], the amorphous colloidal silica exhibited very high stability in acidic media, which is in accordance with the high zeta potential of NS suspension. Whereas, when the pH was in the range of around 7–11, both basic (Si–O−) and acidic (Si–OH) groups could exist on the silica particle surfaces simultaneously [28]. They were capable of forming acid–base bonds (Si–OH….−O–Si) between adjacent particles, followed by a transformation into stable siloxane bonds (Si–O–Si). Thus, this mechanism could facilitate the coagulation of silica gel, negatively affecting the stability of dispersion in the alkaline environment [28]. Therefore, since the pH values of both MS and NS/MS suspensions lie in the range of 7–11, the zeta potentials and the suspension stability were lowered in comparison to the NS solution. In addition, the conductivity of MS suspension was measured to be 2523 μS/cm, which is higher than that of NS suspension (1370 μS/cm). This was ascribed to abundant impurity ions and resultant higher ionic concentration in MS suspension.
3.2 Cyclic voltammetry
Figure 1 presented the CV curves of NS, MS, and NS/MS suspensions in the voltages ranging from 0 to 1 V and from −1 to 1 V. For each parameter, the second cycles during scanning were selected as the representative CV profiles under consideration that the first cycles were not complete. The full data of the CV can be found in the Fig. S2.
Obviously, a reversible electronic double-layer capacitance (EDLC) behavior can be observed in NS and NS/MS dispersions [29, 30]; see Fig. 1a, b. These profiles displayed the shape of a parallelogram and they also exhibited mirror images with respect to the zero-current line. This indicated that those negatively charged silica particles were electrosorbed onto CF surfaces during the forward scanning; while in the backward scanning process, the colloids could reversibly detach from the positive fiber electrode.
Furthermore, for the NS and NS/MS dispersions, minor oxidation was registered in the CV curves at about 1 V. In the voltage range of 0–1 V, the CV curves of the NS/MS electrolyte showed a larger integral area (AreaNS/MS = 0.035) compared with that of NS suspension (0.018), demonstrating higher specific capacitances. Despite similar pH values between the two samples, NS/MS dispersion could possess higher ionic concentration and higher conductivity due to the addition of MS particles in comparison to NS electrolyte, which might contribute to the larger capacitances [31, 32].
For the MS suspension, the CV profile presented a pronounced oxidation reaction at around 1 V owing to the electrochemical oxidation of CF itself in alkaline media [33]. As a result, abundant oxygen-rich functional groups, such as carbonyl, carboxyl, hydroxyl, etc., were generated on the fiber surfaces [33,34,35]. These introduced groups benefited the improvement of adhesion between fiber and matrix, as discussed in Sect. 3.7. Additionally, MS electrolyte showed obviously larger integration areas enveloped by the CV curves (AreaMS = 0.070) in comparison to other suspensions, which suggested larger specific capacitance. This difference in the capacitance behavior among these suspensions might be ascribed to the chemical interaction between the aqueous electrolytes and the surface functionalities of CF as well as the types of electrolytes [36,37,38,39,40]. Additionally, smaller particle sizes (cf. Fig. S1) and the resultant higher surface area of MS may strongly correlate to the larger specific capacitance.
3.3 Morphological analysis
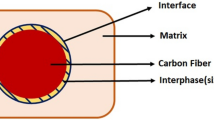
Figure 2 shows the surface morphological features of virgin CFs presenting smooth and neat surfaces with regular, longitudinal striations and gullies.
After EPD modification with NS, quite a few NS agglomerates with diameters up to several micrometers were electro-sorbed onto fiber surfaces; see Fig. 3a, b. These agglomerated NS structures were uniformly distributed on each filament, indicating a successful deposition of extremely fine particles at the nano-level under an applied electric field. This approach provided a promising solution for overcoming the penetration difficulty of NS inside a carbon yarn induced by extensive agglomeration [22].
Figure 3c, d presented that the micro-sized silica colloids were well deposited onto CFs. In comparison to NS coatings, MS layers with less agglomeration were more uniform and they covered large areas of fiber surfaces. Also, every single fiber was capable of being decorated with MS fines during the EPD process, revealing a stable and reliable coating quality. This can be mainly explained by the smaller particle sizes of MS, as demonstrated above.
Figure 3e, f presented the CF surfaces coated with hybrid NS/MS particles. A large amount of agglomerated silica structures with sizes up to several micrometers were found on each filament. In comparison to NS-modified CFs, these well-deposited silica agglomerates exhibited coarser structures. Some bare surface areas can still be observed between adjacent agglomerates.
Figure 4 exhibited the surfaces of modified CFs after being immersed in the cementitious pore solution for 28 days. For NS-modified CFs after exposure to cement pore solution for 28 days, some finely structured hydration products emerged on the CFs; see Fig. 4a, b. These mineral structures, identified as calcium silicate hydrate (C–S–H) and calcite in prior investigations [10], are tightly bonded to the fiber surfaces, thereby contributing to the enhancement of bonding at the fiber-cement interface.
Figure 4c, d presented the fiber surfaces modified with MS after 28 days of immersion in pore solution. It can be visible that the MS-coated samples showed a large amount of mineral structures. The irregular, fine-structured cement hydration products exhibited a similar appearance to that of NS-modified samples. Hence, it can be reasonably inferred that C–S–H gel could also be formed on the surfaces of MS-modified fibers due to the pozzolanic reaction between amorphous MS particles and Ca(OH)2; as seen in the following reaction mechanism [41]:
Moreover, a crystalline structure was found on the fiber surface (see Fig. 4c), which was considered to be calcite (CaCO3) according to its regular radial shape. The generation of calcium carbonate was further proved in the XRD and TGA parts.
Figure 4e, f showed that abundant fine hydration products emerged on the surfaces of CFs treated in NS/MS suspension. Meanwhile, some large pieces of mineral structures can be observed on fiber surfaces and the sample holder, revealing that these coarse-grained mineral structures weakly bonded onto fiber surfaces can be easily formed due to the agglomeration of hybrid NS/MS particles. Additionally, a regular spherical structure was observed in Fig. 4e, which exhibited the typical radial shape of calcite. The formed mineral structures after immersion in pore solution were proved to be C–S–H gel and calcite crystals (see XRD and TGA results).
3.4 X-ray diffraction (XRD)
Figure 5 presented that all the samples possess a major reflex at the scattering angle (2θ) of approximately 25° and a lower peak at about 44°, which was typically assigned to the (002) layer plane of disordered graphite and (100) graphitic plane [42, 43], respectively. The characteristic values of crystalline geometry including thickness (\({L}_{c}\)), layer plane length (\({L}_{a}\)), and interlayer spacing (\({d}_{002}\)) can be derived according to Bragg and Debye–Scherrer equations [44,45,46]:
where \(\theta \) represents one-half of scattering angle (\(2\theta \)) in radians, \(\lambda \) (= 0.15418 nm) indicates the X-rays’ wavelength [46], \(\beta \) indicates the full width at half-maximum (FWHM) of (100) and (002) peaks in radians [47], and \(K\) is the form factor, which is 1.84 for (100) layer plane [44] and 0.9 for (002) graphitic plane [45].
Figure 6a suggested that the mean d-spacing of the 002 reflections can be reduced during EPD treatment, indicating a higher degree of layer plane stacking (see Fig. 8), consistent with the previous investigation [18]. Also, no significant difference in \({d}_{002}\) values between treated samples were registered. A plausible explanation is that some defects might be removed during the EPD treatment, leading to more graphitic structures [48,49,50]. Figure 6b exhibited that EPD treatment resulted in an improvement of crystallite thickness, which has a good agreement with the previous work [18]. Both \({L}_{c}\) values and \({d}_{002}\) spacing are strongly correlated with the change of fiber diameters. Figure 6c presented the diameters of original and modified CFs, revealing a general descending tendency after EPD surface treatment. For each parameter, at least 11 fibers were measured based on microscopic observations. For modified samples, the diameters showed a strong positive correlation with the crystallite thickness; see Fig. 6d. The augmentation of \({L}_{c}\) value appears to be the dominant contributor to the increase in the width of EPD-treated fibers. Notably, the diameter of NS-modified CF was slightly increased in comparison to the Ref sample, since the NS-CF possessed the largest crystallite thickness (\({L}_{c}\)) among all the samples, thus raising fiber size despite the decline in \({d}_{002}\) interlayer spacing. Whereas, MS-CF and NS/MS-CF specimens demonstrated a diminution of fiber diameters compared to untreated CFs, this can be attributed to the lesser extent of increase in the thickness of graphite layers compared to the NS-CF sample. Consequently, this cannot compensate for the loss of interlayer distance.
Figure 7a displayed that the value of \({L}_{a}\) was diminished after EPD modification, demonstrating a decline in lengths of graphite layer planes and fracture of the crystallites of the fiber surface [51]. This decrease was strongly correlated to the general descending tendency of fiber strength, cf. Sect. 3.6. Figure 7b disclosed that the \({L}_{a}\) values were lowered as the pH values increased. This phenomenon can be explained by the addition of more MS particles, which increases the pH and electrical conductivity due to the presence of alkali oxides in MS, as aforementioned (Fig. 8). Therefore, the electrochemical etching might be intensified, provoking the splitting of graphite crystallites and decrement of \({L}_{a}\) [52]. Based on the findings, it can be concluded that EPD treatment significantly influences the microstructures of CFs.
Figure 9 showed the XRD curves of the untreated and modified CFs immersed in cementitious pore solution for 28 days. For reference CFs, no other characteristic peaks appeared in the XRD profile except for (002) and (100) reflections. Obviously, four minor peaks can be recognized in the XRD patterns for other samples modified with NS and/or MS at around 29.6°, 36.2°, 39.6°, and 43.4°, respectively, which corresponded to the (104), (110), (113), and (202) reflections of calcite crystallite, respectively [53]. The formation of calcite microcrystallite can be attributable to the nucleation effect of small silica particles in the pore solution [41]. Meanwhile, the calcium ions (Ca2+) can be chemisorbed by the weakly acidic silanol groups of silica particles due to the reaction between Si–OH groups and Ca(OH)2 [27], which may also benefit the generation of calcium carbonate.
3.5 Thermo-gravimetric analysis (TGA)
The temperature resistance of pristine CFs as well as the electrophoretically treated fibers before and after being stored in cement pore solution was tested by TGA. Derivative thermogravimetry (DTG) was also employed to study the weight loss rate of samples as a function of the temperature. It was shown that the reference sample was oxidized initially at 675 °C with 20% weight loss and then completely oxidized at the temperature of approximately 837 °C; see Fig. 10.
After EPD modification, the CFs coated with NS, MS, and NS/MS showed a 20% weight loss at temperatures reaching 667 °C, 663 °C, and 664 °C, respectively, complete oxidation up to temperatures of about 803 °C, 845 °C, and 816 °C, as seen in Fig. 10a. The modified fibers presented a slight decline in thermal stability compared with pristine CFs, which could be owed to the fibers’ mild oxidation during electrochemical treatment [10, 54]. Interestingly, in the case of complete burning-off, the MS-CF sample exhibited the highest temperature among all the specimens; meanwhile, the samples of MS-CF and NS/MS-CF still maintained a large mass of about 4% after complete oxidation. This residual material could be the large amounts of silica particles deposited neighboring the fibers, indicating the effective attachment of massive silica during EPD.
Figure 10c showed their corresponding DTG profiles, where the minimum peaks were registered at 797 °C for Ref, 767 °C for NS-CF, 755 °C for MS-CF, and 766 °C for NS/MS-CF, respectively, demonstrating the lowered temperature stability of modified CFs again. The relationship of temperatures at DTG peaks with the value of \({L}_{a}\) and pH was given in Fig. 11. Figure 11a presented that the temperatures at DTG peak were positively associated with the length of the graphite layer, revealing that the diminution of crystalline sizes could adversely affect the thermo-stability of the fiber itself. While, for modified samples, the pH values exhibited a negative correlation with the temperatures at DTG peaks (Fig. 11b), which is owed to the negative pH dependence of \({L}_{a}\) (cf. Figure 7b).
Figure 10b, d showed the TGA and DTG results for the plain CFs as well as modified samples immersed in pore solution for 28 days. In comparison to the Ref sample, the treatment of NS-CF/28d, MS-CF/28d, and NS/MS-CF/28d reduced the temperatures of 20% weight loss to 635 °C, 630 °C, and 637 °C, respectively, and that of complete burning-off down to 784 °C, 795 °C, and 820 °C, respectively; see Fig. 10b. This diminution of temperature stability can be ascribed to the fibers’ slight oxidative damage after being stored in alkaline media, i.e., cement pore solution [55, 56], as reported in the previous investigations [10, 17].
From the DTG outcome in Fig. 10d, additional sharp peaks or remarkable humps can be pronouncedly recognized at around 650 °C for each curve of treated samples. These peaks typically represented the decomposition of CaCO3 [7], disclosing the existence of calcium carbonate crystallites onto the modified fibers. The generation of calcite is on account of the nucleating effect of silica particles (Fig. 11). Besides, low peaks were registered between 30 and 170 °C for the samples treated in NS/MS suspension, which can be mainly traced back to the dehydration of C–S–H and ettringite (AFt) [57].
3.6 Tensile strength and modulus of electrophoretically modified CF
The changes in axial tensile properties of EPD-treated CFs were given in Fig. 12. In comparison to the reference specimen, the tensile strengths of NS-CF, MS-CF, and NS/MS-CF samples were reduced by 5%, 8%, and 2%, respectively; see Fig. 12a. This slight diminution of fiber strengths after EPD treatment was also observed in the previous work [10], which may arise from the fibers’ oxidation and the carbon loss during modification [58]. Interestingly, the fiber strengths are proportional to the \({L}_{a}\) value (see Fig. 12c), suggesting a linear dependence between tensile strengths and layer plane length. This correlation between strength and \({L}_{a}\) was also reported by Li et al. [52]. Accordingly, this refinement of graphitic crystallites induced by electrochemical etching appears to negatively impact the mechanical strength of CFs. However, a study exists indicating independence between two variables [59]. Thus, further investigations are needed to elucidate their relationship.
Figure 12b suggested that the electrophoretic treatment had a slight negative impact on the fiber modulus. Compared with untreated samples, the modulus of CF was lowered by 8% for NS-CF, 11% for MS-CF, and 6% for NS/MS-CF, respectively. This general decline of tensile modulus due to the EPD treatment aligns with the published studies by authors [10, 18]. As seen in Fig. 12d, the EPD modification likely leads to the fracture of crystallites inside CFs, consequently resulting in a decline in modulus. In a word, the tensile strength and modulus of CFs could be degraded to only a minor extent by the EPD treatment, which may not significantly hinder the application of modified fibers.
3.7 Pullout behavior of electrophoretically modified CF
Figure 13a exhibited the representative force–displacement pullout curves for pristine and treated CF. The EPD treatment was able to impart a better adhesion to the CF/cement interface, as manifested by the obviously higher pullout forces. It is quite evident that the MS-CF showed the most superior bonding between fiber and matrix, followed by the NS-CF and NS/MS-CF samples.
a Representative force–displacement pullout curves as well as the outcome of b apparent interfacial shear strength \({\tau }_{app}\) and frictional bond strength \({\tau }_{fr}\), c chemical debonding energy \({G}_{d}\), d total pullout energy \({E}_{t}\) and slippage energy \({E}_{s}\) for untreated samples and modified CFs
To comprehensively evaluate the anchorage effect of the fiber-matrix interface, an interfacial parameter—apparent interfacial shear strength—is most frequently used, and it can be obtained by dividing the maximum force \({F}_{max}\) by the contact area between CF and cement [60], as defined in Eq. (4).
where \({d}_{f}\) is the diameter of CF and \({L}_{e}\) is the fiber-embedded length in the matrices.
For evaluating the frictional interaction between fiber and matrix, the maximal frictional force \({F}_{fr}\) at the beginning of the slippage stage is utilized to calculate the frictional bond strength \({\tau }_{fr}\), seen as follows [3]:
The chemical debonding energy can be obtained by using Eq. (6) [12, 61], as described below.
where \({E}_{f}\) is Young’s modulus of CF.
Additionally, the capacity of resisting being pullout is comprehensively assessed by calculating the energy dissipation per unit area at the fiber/cement interface during the whole pullout process. Thus, the total pullout energy \({E}_{t}\) was defined as follows [8]:
To evaluate the sliding resistance during pullout, an indicator of slippage energy \({E}_{s}\) is used. It can be obtained by dividing a specific frictional work \({W}_{f}\) by the contact area at the interface [8]:
More details of the interfacial parameters mentioned above can be found in the previous work [8, 12].
As shown in Fig. 13b, the maximum shear strengths \({\tau }_{app}\) of CFs modified with NS, MS, and NS/MS were augmented by 104%, 125%, and 71%, respectively, in comparison to the reference group. The results of \({\tau }_{fr}\) showed a similar tendency to that of apparent bond strength. Obviously, the untreated CF suffered a poor bonding toward the adjacent matrices owing to its hydrophobic surfaces; see Fig. 14a.
The augmentation of interfacial bonding for NS-modified samples can be explained that the C–S–H gel and calcite crystallites were able to grow in the peripheral zone of the fiber owing to the pozzolanic reaction and nucleating effect of NS particles deposited [10], as discussed above. These substantial, tightly-bonded mineral structures conduced to the improvement of mechanical interaction at the interface; see Fig. 4a. Besides, the NS coatings attached to fiber surfaces are hydrophilic due to the Si–OH groups, improving the wettability of CFs. Thus, both physical and chemical interaction between modified CFs and cement matrices were remarkably enhanced, cf. Fig. 13b, c.
The MS-CF specimen showed the best bond performance among all the groups, which can be mainly attributable to the following reasons: On the one hand, the abundant MS coating material could promote the formation of calcite crystallites and C–S–H gel due to its pozzolanic nature and nucleating action. In comparison to NS coatings, the deposited MS exhibited a larger amount and more homogeneous distribution with fewer agglomerates; thus, leading to a superior bond property. On the other hand, CV measurements manifested the occurrence of oxidation reaction of CFs during EPD treatment in alkaline MS suspension, which is very likely to produce oxygen-related functional groups onto fiber surfaces [33,34,35]. Those groups could chemisorb Ca2+ in the pore solution, forming ionic bonding; meanwhile, hydrogen bonding can be established between –OH functionalities and the oxygen from hydration products, benefiting the chemical adhesion of CF toward cement matrices [8] (see Fig. 14c). Additionally, the CF wettability could be ameliorated owing to the hydrophilicity of the functional groups, densifying the interfacial transition zone (ITZ).
The CFs treated in NS/MS suspension presented a drastic increment of interfacial bonding. As mentioned above, large amounts of C–S–H gel and calcite could be generated onto surfaces of CFs embedded in cement matrix (cf. Figs. 4, 9), which is in favor of improving interfacial interaction between CF and the matrix. Notably, in comparison to NS-CF, although NS/MS-CF exhibited a larger mass of mineral coatings before and after immersion in pore solution (cf. TGA part), it showed relatively lower values of \({\tau }_{app}\), \({\tau }_{fr}\), and \({G}_{d}\). This might be explained by the formation of large, coarse-grained hydration products onto fiber surfaces when embedded in cement matrix, owing to the extensive agglomeration of NS/MS particles (cf. Figs. 3, 4, and 14d). While, these large mineral structures were weakly bonded to the CFs, which cannot offer as effective anchoring as NS-CF samples with mainly fine-structured hydration products.
Notably, the bond behavior is closely related to the length of graphite layers. The Ref sample (with the lowest bond performance) exhibited the highest \({L}_{a}\) value and MS-CF (with the best bonding) showed the smallest crystallite length, revealing some negative correlation between bond strength and crystallite dimensions. Liu et al. [59] stated that the reduction of graphitic crystallite size gave rise to more active carbon atoms at edges, making it easier to produce functional groups. This ultimately favors the enhancement of interfacial shear strength. Hence, in the study at hand, the decrement of \({L}_{a}\) value led to general ascending tendency of bond properties.
The total pullout and slippage energy were used to assess the energy dissipation ability at the fiber-cement interface; see Fig. 13d. The samples modified with NS, MS, and NS/MS showed an obvious augmentation in total pullout energy by 79%, 116%, and 91%, respectively, relative to the untreated CF. A similar tendency was also observed in the outcome of slippage energy. Obviously, the EPD modification of CFs gave rise to an increase in dissipated energy during fiber pullout from cement, which is advantageous for improving the crack-bridging ability for CFRC.
Interestingly, in comparison to the NS-CF sample, NS/MS-CF presented lower \({\tau }_{app}\) and \({\tau }_{fr}\) values, but better results in the total pullout and slippage energy. This phenomenon can be explained by the larger amount of volume-expanded C-S–H generated onto NS/MS-CF according to TGA results (cf. Figure 10b). These massive hydrated products might tailor the compactness of ITZ neighboring fibers and increase the lateral pressure toward CF surfaces, thereby enhancing the resistance to being pulled out. Nevertheless, large pieces of mineral structures can be more easily generated onto NS/MS-CF due to the extensive agglomeration, diminishing the ‘contact points’ between additional hydration products and CF, and thus limiting the further increase of bond strength.
The \({E}_{s}\)/\({E}_{t}\) ratio was calculated to reveal the dominance of the frictional interaction in the whole fiber pullout process. The Ref, NS-CF, and MS-CF samples possess similar values of below 0.80, while the NS/MS-CF specimen has the highest proportion of friction mechanism with values of about 0.82. This suggests that for all the samples, the frictional mechanism accounted for most of the total dissipated energy at the fiber-cement interface. In particular, friction played a more significant role in the crack-bridging action for the NS/MS-CF sample compared to other specimens. This could be attributed to its relatively weak chemical bonding, as shown in Fig. 13c.
4 Summary and conclusions
The surfaces of CFs were optimized by using an electrophoretic deposition approach (EPD) with nano-silica (NS), micro-silica (MS), and the hybrid NS/MS particles to improve the poor bonding between CFs and cement-based matrices. Silica particles were successfully deposited onto fiber surfaces under an applied electric field, as confirmed by electron microscopy. The impact of EPD treatment on the physical properties of CFs and the reaction kinetics of CF electrodes were comprehensively investigated. Single-fiber pullout experiments were also carried out to assess the interfacial adhesion at the fiber-cement interface. The following conclusions can be drawn:
-
1.
Zeta measurements showed that the silica particles stably dispersing in the aqueous media possessed a negative charge, which enabled the movement of these particles during the EPD process. In comparison to the NS regime, a slight decrease of zeta potentials was recorded for MS, and NS/MS suspensions, which may be due to some coagulation of silica colloids in the neutral and alkaline conditions. The EPD systems for NS and NS/MS suspensions act as ideal reversible capacitors, while some oxidation reactions were registered during treatment in MS electrolyte, as proved by CV analysis.
-
2.
EPD treatment considerably decreased the interlayer d-spacing and raised the crystallite thickness \({L}_{c}\) of CFs, which strongly influenced the fiber diameters. Also, the layer plane length of graphitic crystallites was reduced by surface modification, which could bring about a diminution of thermal stability and a descending trend in tensile strength and modulus.
-
3.
ESEM observation and XRD results show that a substantial amount of amorphous silica colloids were deposited onto CFs, which could form C-S–H gel and calcite crystallites in a cementitious environment owing to their pozzolanic reactivity and nucleating effects. The formed fine mineral structures could densify the interfacial transition zones between fiber and cementitious matrix. Additionally, electrooxidation took place for MS-CF samples. The resultant formation of oxygen-containing groups brought about stronger chemical bonds at the fiber/cement interface, leading to superior bond performance. Interestingly, for NS/MS-CF samples with extensive agglomerates, large pieces of hydration products can be more easily formed when immersed in cement pore solution. This impaired the anchorage effect but benefited the improvement of energy dissipation ability compared with the NS-CF sample. As well, among all the groups, the NS/MS coated CF exhibited the highest dominance of frictional interaction throughout the fiber pullout process.
In this article, EPD was demonstrated to be a simply-operated, energy-efficient technology for modifying CF surfaces. Considering the high cost of NS manufacture and the excellent pullout outcome of MS-CF, the silica fume reclaimed from industrial wastes was recommended to serve as a deposit in the EPD modification. To study the influence of amorphous carbon on the physio-chemical properties of CFs, further investigation using characterization methods such as Raman and XPS analysis is needed. Future studies should also explore other organic materials to optimize the ITZ between CF and matrices and expand the engineering applications of CF material. Further investigations on the role of altered surface wettability of modified CF in relation to the workability of fiber-added cementitious composites are also necessary for future studies.
References
Li H, Wang L, Zhang Y, Yang J, Tsang DC, Mechtcherine V (2023) Biochar for sustainable construction industry. Current Developments in Biotechnology and Bioengineering, Elsevier2023, pp 63–95
Environment U, Scrivener KL, John VM, Gartner EM (2018) Eco-efficient cements: potential economically viable solutions for a low-CO2 cement-based materials industry. Cem Concr Res 114:2–26
Li H, Yang J, Wang L, Li L, Xia Y, Köberle T, Dong W, Zhang N, Yang B, Mechtcherine V (2023) Multiscale assessment of performance of limestone calcined clay cement (LC3) reinforced with virgin and recycled carbon fibers. Constr Build Mater 406:133228
Poudyal L, Adhikari K (2021) Environmental sustainability in cement industry: an integrated approach for green and economical cement production. Resour Environ Sustain 4:100024
C.T.E.C. Association, Activity report, Brussels (2020)
Vieira DR, Calmon JL, Coelho FZ (2016) Life cycle assessment (LCA) applied to the manufacturing of common and ecological concrete: a review. Constr Build Mater 124:656–666
Li H, Liebscher M, Ranjbarian M, Hempel S, Tzounis L, Schröfl C, Mechtcherine V (2019) Electrochemical modification of carbon fiber yarns in cementitious pore solution for an enhanced interaction towards concrete matrices. Appl Surf Sci 487:52–58
Li H, Liebscher M, Zhao D, Yin B, Du Y, Yang J, Kaliske M, Mechtcherine V (2022) A review of carbon fiber surface modification methods for tailor-made bond behavior with cementitious matrices. Prog Mater Sci 132:101040
Li H, Liebscher M, Michel A, Quade A, Foest R, Mechtcherine V (2021) Oxygen plasma modification of carbon fiber rovings for enhanced interaction toward mineral-based impregnation materials and concrete matrices. Constr Build Mater 273:121950
Li H, Liebscher M, Curosu I, Choudhury S, Hempel S, Davoodabadi M, Dinh TT, Yang J, Mechtcherine V (2020) Electrophoretic deposition of nano-silica onto carbon fiber surfaces for an improved bond strength with cementitious matrices. Cement Concr Compos 114:103777
Lu Z, Hanif A, Sun G, Liang R, Parthasarathy P, Li Z (2018) Highly dispersed graphene oxide electrodeposited carbon fiber reinforced cement-based materials with enhanced mechanical properties. Cement Concr Compos 87:220–228
Li H, Zhao D, Liebscher M, Yin B, Yang J, Kaliske M, Mechtcherine V (2022) An experimental and numerical study on the age depended bond-slip behavior between nano-silica modified carbon fibers and cementitious matrices. Cement Concr Compos 128:104416
Raphael N, Namratha K, Chandrashekar B, Sadasivuni KK, Ponnamma D, Smitha A, Krishnaveni S, Cheng C, Byrappa K (2018) Surface modification and grafting of carbon fibers: a route to better interface. Prog Cryst Growth Charact Mater 64(3):75–101
Boccaccini AR, Cho J, Roether JA, Thomas BJ, Minay EJ, Shaffer MS (2006) Electrophoretic deposition of carbon nanotubes. Carbon 44(15):3149–3160
Huang L, Chen D, Ding Y, Feng S, Wang ZL, Liu M (2013) Nickel–cobalt hydroxide nanosheets coated on NiCo2O4 nanowires grown on carbon fiber paper for high-performance pseudocapacitors. Nano Lett 13(7):3135–3139
Li H, Liebscher M, Yang J, Davoodabadi M, Li L, Du Y, Yang B, Hempel S, Mechtcherine V (2022) Electrochemical oxidation of recycled carbon fibers for an improved interaction toward alkali-activated composites. J Clean Prod 368:133093
Li H, Liebscher M, Ly KH, Ly PV, Köberle T, Yang J, Fan Q, Yu M, Weidinger IM, Mechtcherine V (2022) Effect of electrophoretic deposition of micro-quartz on the microstructural and mechanical properties of carbon fibers and their bond performance toward cement. J Mater Sci 57(48):21885–21900
Li H, Liebscher M, Micusik M, Yang J, Sun B, Yin B, Yu M, Mechtcherine V (2022) Role of pH value on electrophoretic deposition of nano-silica onto carbon fibers for a tailored bond behavior with cementitious matrices. Appl Surf Sci 600:154000
Constantinides G (2013) Nanoscience and nanoengineering of cement-based materials. Nanotechnology in Eco-Efficient Construction, Elsevier2013, pp 9–37a
Lei D-Y, Guo L-P, Sun W, Liu J, Shu X, Guo X-L (2016) A new dispersing method on silica fume and its influence on the performance of cement-based materials. Constr Build Mater 115:716–726
Black L (2016) Low clinker cement as a sustainable construction material. Sustainability of Construction Materials, 415–457
Nadiv R, Peled A, Mechtcherine V, Hempel S, Schroefl C (2017) Micro-and nanoparticle mineral coating for enhanced properties of carbon multifilament yarn cement-based composites. Compos B Eng 111:179–189
Signorini C, Nobili A, Gonzalez EC, Siligardi C (2018) Silica coating for interphase bond enhancement of carbon and AR-glass textile Reinforced Mortar (TRM). Compos B Eng 141:191–202
Castro Y, Ferrari B, Moreno R, Duran A (2004) Coatings produced by electrophoretic deposition from nano-particulate silica sol–gel suspensions. Surf Coat Technol 182(2–3):199–203
Li H, Schamel E, Liebscher M, Zhang Y, Fan Q, Schlachter H, Köberle T, Mechtcherine V, Wehnert G, Söthje D (2023) Recycled carbon fibers in cement-based composites: influence of epoxide matrix depolymerization degree on interfacial interactions. J Clean Prod 411:137235
A. Standard, Zeta potential of colloids in water and waste water, ASTM Standard D (1985) 4187-82
Greenberg SA (1956) The chemisorption of calcium hydroxide by silica. J Phys Chem 60(3):325–330
Depasse J, Watillon A (1970) The stability of amorphous colloidal silica. J Colloid Interface Sci 33(3):430–438
Pell W, Conway B, Marincic N (2000) Analysis of non-uniform charge/discharge and rate effects in porous carbon capacitors containing sub-optimal electrolyte concentrations. J Electroanal Chem 491(1–2):9–21
Xu B, Wu F, Chen S, Zhang C, Cao G, Yang Y (2007) Activated carbon fiber cloths as electrodes for high performance electric double layer capacitors. Electrochim Acta 52(13):4595–4598
Tsay K-C, Zhang L, Zhang J (2012) Effects of electrode layer composition/thickness and electrolyte concentration on both specific capacitance and energy density of supercapacitor. Electrochim Acta 60:428–436
Zheng JP, Jow T (1997) The effect of salt concentration in electrolytes on the maximum energy storage for double layer capacitors. J Electrochem Soc 144(7):2417
Bismarck A, Kumru M, Springer J, Simitzis J (1999) Surface properties of PAN-based carbon fibers tuned by anodic oxidation in different alkaline electrolyte systems. Appl Surf Sci 143(1):45–55
Proctor A, Sherwood PM (1983) X-ray photoelectron spectroscopic studies of carbon fibre surfaces—II: The effect of electrochemical treatment. Carbon 21(1):53–59
Alexander M, Jones F (1994) Effect of electrolytic oxidation on the surface chemistry of type a carbon fibres—Part I, X-ray photoelectron spectroscopy. Carbon 32(5):785–794
Fic K, Lota G, Meller M, Frackowiak E (2012) Novel insight into neutral medium as electrolyte for high-voltage supercapacitors. Energy Environ Sci 5(2):5842–5850
Demarconnay L, Raymundo-Piñero E, Béguin F (2010) A symmetric carbon/carbon supercapacitor operating at 1.6 V by using a neutral aqueous solution. Electrochem Commun 12(10):1275–1278
Hu C-C, Wang C-C (2004) Effects of electrolytes and electrochemical pretreatments on the capacitive characteristics of activated carbon fabrics for supercapacitors. J Power Sources 125(2):299–308
Andreas HA, Conway BE (2006) Examination of the double-layer capacitance of an high specific-area C-cloth electrode as titrated from acidic to alkaline pHs. Electrochim Acta 51(28):6510–6520
Karamanova B, Stoyanova A, Shipochka M, Veleva S, Stoyanova R (2020) Effect of alkaline-basic electrolytes on the capacitance performance of biomass-derived carbonaceous materials. Materials (Basel) 13(13)
John E, Matschei T, Stephan D (2018) Nucleation seeding with calcium silicate hydrate: a review. Cem Concr Res 113:74–85
Qian X, Zhi J, Chen L, Zhong J, Wang X, Zhang Y, Song S (2018) Evolution of microstructure and electrical property in the conversion of high strength carbon fiber to high modulus and ultrahigh modulus carbon fiber. Compos A Appl Sci Manuf 112:111–118
Wang S, Chen Z-H, Ma W-J, Ma Q-S (2006) Influence of heat treatment on physical–chemical properties of PAN-based carbon fiber. Ceram Int 32(3):291–295
Cullity BD (1956) Elements of X-ray diffraction. Addison-Wesley Publishing
Jeffery JW (1971) Methods in X-ray crystallography. Academic Press, London
Anderson DP (1991) Carbon fiber morphology. 2. Expanded wide-angle X-ray diffraction studies of carbon fibers. Dayton Univ OH Research Inst
Epp J (2016) X-ray diffraction (XRD) techniques for materials characterization. Materials characterization using nondestructive evaluation (NDE) methods, Elsevier, pp 81–124
Adams P, Katzman H, Rellick G, Stupian G (1998) Characterization of high thermal conductivity carbon fibers and a self-reinforced graphite panel. Carbon 36(3):233–245
Own S-H, Subramanian R, Saunders S (1986) A bimodal lognormal model of the distribution of strength of carbon fibres: effects of electrodeposition of titanium di (dioctyl pyrophosphate) oxyacetate. J Mater Sci 21(11):3912–3920
Qiu L, Zheng X, Zhu J, Su G, Tang D (2013) The effect of grain size on the lattice thermal conductivity of an individual polyacrylonitrile-based carbon fiber. Carbon 51:265–273
Jiang G, Pickering SJ (2016) Structure–property relationship of recycled carbon fibres revealed by pyrolysis recycling process. J Mater Sci 51:1949–1958
Li Z, Wang J, Tong Y, Xu L (2012) Anodic oxidation on structural evolution and tensile properties of polyacrylonitrile based carbon fibers with different surface morphology. J Mater Sci Technol 28(12):1123–1129
Li T, Sui F, Li F, Cai Y, Jin Z (2014) Effects of dry grinding on the structure and granularity of calcite and its polymorphic transformation into aragonite. Powder Technol 254:338–343
Bismarck A, Pfeifer G, Springer J (2000) Study on surface-and mechanical fiber characteristics and their effect on epoxy composite properties tuned by continuous anodic carbon fiber oxidation. J Adhes Sci Technol 14(5):661–690
Sugama T, Kukacka L, Carciello N, Galen B (1988) Oxidation of carbon fiber surfaces for improvement in fiber-cement interfacial bond at a hydrothermal temperature of 300° C. Cem Concr Res 18(2):290–300
Wang Y, Zhang S, Li G, Shi X (2019) Effects of alkali-treated recycled carbon fiber on the strength and free drying shrinkage of cementitious mortar. J Clean Prod 228:1187–1195
Schöler A, Lothenbach B, Winnefeld F, Zajac M (2015) Hydration of quaternary Portland cement blends containing blast-furnace slag, siliceous fly ash and limestone powder. Cement Concr Compos 55:374–382
Kozlowski C, Sherwood PM (1985) X-ray photoelectron-spectroscopic studies of carbon-fibre surfaces. Part 5.—The effect of pH on surface oxidation. J Chem Soc Faraday Trans 1: Phys Chem Condens Phases 81(11):2745–2756
Liu J, Tian Y, Chen Y, Liang J (2010) Interfacial and mechanical properties of carbon fibers modified by electrochemical oxidation in (NH4HCO3)/(NH4) 2C2O4· H2O aqueous compound solution. Appl Surf Sci 256(21):6199–6204
Katz A, Li VC, Kazmer A (1995) Bond properties of carbon fibers in cementitious matrix. J Mater Civ Eng 7(2):125–128
Redon C, Li VC, Wu C, Hoshiro H, Saito T, Ogawa A (2001) Measuring and modifying interface properties of PVA fibers in ECC matrix. J Mater Civ Eng 13(6):399–406
Acknowledgements
The authors acknowledge the financial support of the Saxon State Parliament. The measurement of the grain size distribution of powders was kindly conducted by Dipl.-Ing. A. Willomitzer, and XRD measurements by Dipl.-Krist. I. Noack.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, H., Liebscher, M., Yang, J. et al. Influence of electrophoretic deposition of micro- or nanosized silica particles on the microstructure of carbon fibers and their bond behavior with cementitious matrices. Mater Struct 57, 107 (2024). https://doi.org/10.1617/s11527-024-02355-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1617/s11527-024-02355-5