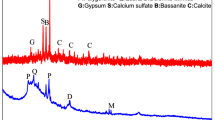
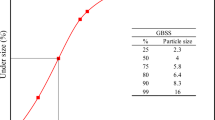
Abstract
This research determines an adequate alkali-activated material (AAM) for the incorporation of huge amounts (20 or 40% vol) of low viscosity organic liquids (LVOL), e.g. for waste stabilization/solidification. The selected AAM are either based on high-Ca content blast furnace slag, or on low Ca-content metakaolin, i.e. on a geopolymer matrix. First, the selection of the AAM is performed to ensure no LVOL leakage and a sufficient compressive strength fc (> 8 MPa). Surfactants are compulsory to allow incorporation. After 90 days curing, for slag pastes, fc ranges between 10 and 20 MPa at 20% vol LVOL, but it is zero at 40% LVOL, whatever the surfactant. For geopolymer pastes, the AAM-LVOL composites have an average fc of 25 MPa at 20% vol LVOL, and of 15 MPa at 40% LVOL. With surfactant, the AAM solid pore structure of slag pastes is denser (with smaller specific surface area and micropore amount); it is unchanged for geopolymer pastes. Whatever the surfactant, air entrained bubbles are present. Their proportion is maximal with Glucopon. Together with LVOL presence, this generally contributes to decreasing fc. The emulsion (entrained air + LVOL droplets) is characterized in hardened AAM by combining 2D Scanning Electron Microscopy and 3D X Ray micro-computed tomography. Surfactants significantly decrease the emulsion droplet size distribution. For geopolymer pastes up to 40% vol LVOL, the most adequate surfactants are Brij O10 and CTAB; for slag paste up to 20% vol LVOL, it is CTAB. Moreover, the setting reactions are not impacted by LVOL or surfactants, and neither are the reaction products. It is concluded that the decrease in mechanical performance of AAM-LVOL composites is only due to physical reasons, particularly the decrease in AAM proportion, the emulsion quality (coalescence, droplet size and shape) and air entrained bubbles.











Similar content being viewed by others
Notes
Coalescence of emulsion droplets means that two individual droplets merge and form a single larger droplet ([20]. The asymptotic case is the complete separation of the emulsion into two distinct liquid phases (here AAM and OL).
The so-called Pickering effect occurs in an aqueous phase loaded with solid particles, where an oil emulsion is also present. It is a mechanism where solid particles are partially wetted by the oil phase and by the aqueous phase. The solid particles accumulated at the oil/water interface contribute to stabilize the emulsion ([20].
A macroscopic void is observed in the GEO paste containing 40%vol. of LVOL + surfactant, due to air incorporation during mixing. For this paste, the addition of surfactant leads to a strong increase in viscosity. For further composite manufacturing, this will require vibrating the mold, to remove any such void.
References
IAEA International Atomic Energy Agency (1992) Treatment and conditioning of radioactive organic liquids. TECDOC 656
Cantarel V, Nouaille F, Rooses A, Lambertin D, Poulesquen A, Frizon F (2015) Solidification/stabilisation of liquid oil waste in metakaolin-based geopolymer. J Nucl Mater 464:16–19. https://doi.org/10.1016/j.jnucmat.2015.04.036
Cantarel V, Lambertin D, Poulesquen A, Leroux F, Renaudin G, Frizon F (2018) Geopolymer assembly by emulsion templating: emulsion stability and hardening mechanisms. Ceram Int 44(9):10558–10568. https://doi.org/10.1016/j.ceramint.2018.03.079
Cuccia V, Freire CB, Ladeira ACQ (2020) Radwaste oil immobilization in geopolymer after non-destructive treatment. Prog Nucl Energy 122:103246. https://doi.org/10.1016/j.pnucene.2020.103246
Almabrok MH, McLaughlan R, and Vessalas K (2011) Investigation of oil solidification using direct immobilization method, presented at the Environmental Research Event, Sydney, Australia
Clark DE, Colombo P, Neilson RM (1982) Solidification of oils and organic liquids. Nucl Waste Manag. https://doi.org/10.2172/6462993
Eddhahak A, Drissi S, Colin J, Caré S, Neji J (2014) Effect of phase change materials on the hydration reaction and kinetic of PCM-mortars. J Therm Anal Calorim 117(2):537–545. https://doi.org/10.1007/s10973-014-3844-x
Masrulita M, Burhan P, Trihadiningrum Y (2018) Stabilization/solidification of waste containing heavy metals and hydrocarbons using OPC and land tras cement. J Ecol Eng 19(6):88–96. https://doi.org/10.12911/22998993/92926
Almabrok MH, McLaughlan RG, Vessalas K, Thomas P (2019) Effect of oil contaminated aggregates on cement hydration. Am J Eng Res 8(5):81–89
Ahdaya M, Imqam A (2019) Investigating geopolymer cement performance in presence of water based drilling fluid. J Pet Sci Eng 176:934–942. https://doi.org/10.1016/j.petrol.2019.02.010
El-Naggar MR, El-Sherief EA, Mekhemar HS (2018) Performance of geopolymers for direct immobilization of solvent extraction liquids: metakaolin/LIX-84 formulations. J Hazard Mater 360:670–680. https://doi.org/10.1016/j.jhazmat.2018.08.057
Planel B, Davy CA, Adler PM, Hauss G, Bertin M, Cantarel V, Lambertin D (2020) Water permeability of geopolymers emulsified with oil. Cem Concr Res 135:106108. https://doi.org/10.1016/j.cemconres.2020.106108
Duxson P, Fernández-Jiménez A, Provis JL, Lukey GC, Palomo A, van Deventer JSJ (2007) Geopolymer technology: the current state of the art. J Mater Sci 42(9):2917–2933. https://doi.org/10.1007/s10853-006-0637-z
Provis JL, Bernal SA (2014) Geopolymers and related alkali-activated materials. Annu Rev Mater Res 44(1):299–327. https://doi.org/10.1146/annurev-matsci-070813-113515
Singh B, Ishwarya G, Gupta M, Bhattacharyya SK (2015) Geopolymer concrete: a review of some recent developments. Constr Build Mater 85:78–90. https://doi.org/10.1016/j.conbuildmat.2015.03.036
Provis JL, van Deventer JSJ (2014) Alkali activated materials. State-of-the-Art Report, RILEM TC 224-AAM
Reeb C, Pierlot C, Davy C, Lambertin D (2020) Incorporation of organic liquids into geopolymer materials - a review of processing, properties and applications. Ceram Int. https://doi.org/10.1016/j.ceramint.2020.11.239
Lambertin D, Rooses A, and Frizon F (2014) Process for preparing a composite material from an organic liquid and resulting material. WO2014/044776 A1
Davy CA, Hauss G, Planel B, Lambertin D (2018) 3D structure of oil droplets in hardened geopolymer emulsions. J Am Ceram Soc. https://doi.org/10.1111/jace.16142
Sun Z, Vollpracht A (2019) One year geopolymerisation of sodium silicate activated fly ash and metakaolin geopolymers. Cem Concr Compos 95:98–110. https://doi.org/10.1016/j.cemconcomp.2018.10.014
Pierlot C, Hu H, Reeb C, Bassetti J, Bertin M, Lambertin D, Davy C, Nardello-Rataj V (2022) Selection of suitable surfactants for the incorporation of organic liquids into fresh geopolymer pastes. Chem Eng Sci. https://doi.org/10.1016/j.ces.2022.117635x
Petlitckaia S, Poulesquen A (2019) Design of lightweight metakaolin based geopolymer foamed with hydrogen peroxide. Ceram Int. https://doi.org/10.1016/j.ceramint.2018.10.021
Dong T, Xie S, Wang J, Zhao G, Song Q (2020) Solidification and stabilization of spent TBP/OK organic liquids in a phosphate acid-based geopolymer. Sci Technol Nucl Install 2020:1–7. https://doi.org/10.1155/2020/8094205
Kligys M, Laukaitis A, Sinica M, Sezemanas G (2007) The influence of some surfactants on porous concrete properties. Math Sci 13:310–316
Ouyang X, Guo Y, Qiu X (2008) The feasibility of synthetic surfactant as an air entraining agent for the cement matrix. Constr Build Mater 22(8):1774–1779. https://doi.org/10.1016/j.conbuildmat.2007.05.002
Yan D et al (2021) Effects and mechanisms of surfactants on physical properties and microstructures of metakaolin-based geopolymer. J Zhejiang Univ Sci A 22(2):130–146. https://doi.org/10.1631/jzus.A2000059
Blyth A, Eiben CA, Scherer GW, White CE (2017) Impact of activator chemistry on permeability of alkali-activated slags. J Am Ceram Soc 100:4848–4859. https://doi.org/10.1111/jace.14996
[Pickering 1907] CXCVI.—Emulsions. J Chem Soc Trans 1907, 91: 2001–2021. Doi: https://doi.org/10.1039/CT9079102001
Haha MB, Le Saout G, Winnefeld F, Lothenbach B (2011) Influence of activator type on hydration kinetics, hydrate assemblage and microstructural development of alkali activated blast-furnace slags. Cem Concr Res 41(3):301–310. https://doi.org/10.1016/j.cemconres.2010.11.016
Peix G, Duvauchelle P, Freud N (2000) X-Ray tomography in material science (Hermes Science, London), Chap 1: 15–27
N. Limodin, T. Rougelot, J. Hosdez, 2013, http://isis4d.univ-lille.fr/
Song Y, Davy CA, Troadec D, Blanchenet A-M, Skoczylas F, Talandier J, Robinet JC (2015) Multi-scale pore structure of COx claystone: towards the prediction of fluid transport. Mar Pet Geol 65:63–82. https://doi.org/10.1016/j.marpetgeo.2015.04.004
Kak AC, Slaney M (1988) Principles of computerized tomographic imaging. IEEE Press, Piscataway, NJ
Kak AC, Slaney M (2001) Principles of computerized tomographic imaging. Soc Ind Appl Math. https://doi.org/10.1137/1.9780898719277
Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T (2012) Fiji: an open-source platform for biological-image analysis. Nat Methods 9(7):676
Münch B, Holzer L (2008) Contradicting geometrical concepts in pore size analysis attained with electron microscopy and mercury intrusion. J Am Ceram Soc 91(12):4059–4067. https://doi.org/10.1111/j.1551-2916.2008.02736.x
Massiot D et al (2002) Modelling one- and two-dimensional solid-state NMR spectra. Magn Reson Chem 40(1):70–76. https://doi.org/10.1002/mrc.984
Behfarnia K, Rostami M (2017) An assessment on parameters affecting the carbonation of alkali-activated slag concrete. J Clean Prod 157:1–9. https://doi.org/10.1016/j.jclepro.2017.04.097
Bulejko P, Bílek V (2017) Influence of chemical additives and curing conditions on the mechanical properties and carbonation resistance of alkali-activated slag composites. Mater Technol 51:49–53. https://doi.org/10.17222/mit.2015.185
Song K-I, Song J-K, Lee BY, Yang K-H (2014) Carbonation characteristics of alkali-activated blast-furnace slag mortar. Adv Mater Sci Eng. https://doi.org/10.1155/2014/326458
MacKenzie KJD, Meinhold RH, Sherriffb BL, Xub Z (1993) 27AIand 25Mg solid-state magic-angle spinning nuclear magnetic resonance study of hydrotalcite and its thermal decomposition sequence. J Mater Chem 3:1263–1269. https://doi.org/10.1039/JM9930301263
Cai J, Xiaopeng L, Jiawei T, Brecht V (2020) Thermal and compressive behaviors of fly ash and metakaolin-based geopolymer. J Build Eng 30:101307. https://doi.org/10.1016/j.jobe.2020.101307
Sun Z, Vollpracht A (2018) Isothermal calorimetry and in-situ XRD study of the NaOH activated fly ash, metakaolin and slag. Cem Concr Res 103:110–122. https://doi.org/10.1016/j.cemconres.2017.10.004
Yao X, Zhang Z, Zhu H, Chen Y (2009) Geopolymerization process of alkali–metakaolinite characterized by isothermal calorimetry. Thermochim Acta 493:49–54. https://doi.org/10.1016/j.tca.2009.04.002
Zhang Z, Hao W, Yingcan Z, Andrew R, Provis JL, Bullen F (2014) Using fly ash to partially substitute metakaolin in geopolymer synthesis. Appl Clay Sci 88–89:194–201. https://doi.org/10.1016/j.clay.2013.12.025
Bernal SA, Provis JL, Volker R, Mejia de Gutierrez R (2011) Evolution of binder structure in sodium silicate-activated slag-metakaolin blends. Cem Concr Compos 33:46–54. https://doi.org/10.1016/j.cemconcomp.2010.09.004
Chithiraputhiran S, Narayanan N (2013) Isothermal reaction kinetics and temperature dependence of alkali activation of slag, fly ash and their blends. Constr Build Mater 45:233–242. https://doi.org/10.1016/j.conbuildmat.2013.03.061
Lei L, Li R, Fuddin A (2020) Influence of maltodextrin retarder on the hydration kinetics and mechanical properties of Portland cement. Cem Concr Compos 114:103774. https://doi.org/10.1016/j.cemconcomp.2020.103774
Kochova K, Schollbach K, Gauvin F, Brouwers HJH (2017) Effect of saccharides on the hydration of ordinary Portland cement. Constr Build Mater 150:268–275. https://doi.org/10.1016/j.conbuildmat.2017.05.149
Schneider N, Stephan D (2016) The effect of d-gluconic acid as a retarder of ground granulated blast-furnace slag pastes. Constr Build Mater 123:99–105. https://doi.org/10.1016/j.conbuildmat.2016.06.127
Chen L, Wang Z, Wang Y, Feng J (2016) Preparation and properties of alkali activated metakaolin-based geopolymer. Materials 9(9):767. https://doi.org/10.3390/ma9090767
Palmero P, Formia A, Antonaci P, Brini S, Tulliani JM (2015) Geopolymer technology for application-oriented dense and lightened materials. Elaboration and characterization. Ceram Int 41(10):12967–12979. https://doi.org/10.1016/j.ceramint.2015.06.140
He P et al (2016) Effects of Si/Al ratio on the structure and properties of metakaolin based geopolymer. Ceram Int 42(13):14416–14422. https://doi.org/10.1016/j.ceramint.2016.06.033
Duxson P, Provis JL, Lukey GC, Separovic F, van Deventer JSJ (2005) Si NMR study of structural ordering in aluminosilicate geopolymer gels. Langmuir 21(7):3028–3036. https://doi.org/10.1021/la047336x
Wan Q, Rao F, Song S, Garcia RE, Estrella RM, Patino CL, Zhang Y (2017) Geopolymerization reaction, microstructure and simulation of metakaolin-based geopolymers at extended Si/Al ratios. Cem Concr Compos 79:45–52. https://doi.org/10.1016/j.cemconcomp.2017.01.014
Kuenzel C, Neville TP, Donatello S, Vandeperre L, Boccaccini AR, Cheeseman CR (2013) Influence of metakaolin characteristics on the mechanical properties of geopolymers. Appl Clay Sci 83–84:308–314. https://doi.org/10.1016/j.clay.2013.08.023
Bonk F, Schneider J, Cincotto MA, Panepucci H (2003) Characterization by multinuclear high-resolution NMR of hydration products in activated blast furnace slag pastes. J Am Ceram Soc 86:1712–1719. https://doi.org/10.1111/j.1151-2916.2003.tb03545.x
Myers RJ, Bernal SA, Nicolas RS, Provis JL (2013) Generalized structural description of calcium−sodium aluminosilicate hydrate gels: the cross-linked substituted tobermorite model. Langmuir. https://doi.org/10.1021/la4000473
Schilling PJ, Butler LG, Roy A, Eaton HC (1994) 29Si and 27Al MAS-NMR of NaOH-activated blast-furnace slag. J Am Ceram Soc 77(9):2363–2368. https://doi.org/10.1111/j.1151-2916.1994.tb04606.x
Schneider J, Cincotto MA, Panepucci H (2001) 29Si and 27Al high-resolution NMR characterization of calcium silicate hydrate phases in activated blast-furnace slag pastes. Cem Concr Res 31(7):993–1001. https://doi.org/10.1016/S0008-8846(01)00530-0
Wang SD, Scrivener KL (2003) 29Si and 27Al NMR study of alkali-activated slag. Cem Concr Res 33(5):769–774. https://doi.org/10.1016/S0008-8846(02)01044-X
Hilbig H, Buchwald A (2006) The effect of activator concentration on reaction degree and structure formation of alkali-activated ground granulated blast furnace slag. J Mater Sci 41(19):6488–6491. https://doi.org/10.1007/s10853-006-0755-7
Albidah A, Alghannam M, Abbas H, Almusallam T, Al-Salloum Y (2021) Characteristics of metakaolin-based geopolymer concrete for different mix design parameters. J Mater Res Technol 10:84–98. https://doi.org/10.1016/j.jmrt.2020.11.104
Haha MB, Lothenbach B, Le Saout G, Winnefeld F (2011) Influence of slag chemistry on the hydration of alkali-activated blast-furnace slag—Part I: effect of MgO. Cem Concr Res 41(9):955–963. https://doi.org/10.1016/j.cemconres.2011.05.002
Bilim C, Atiş CD, Tanyildizi H, Karahan O (2009) Predicting the compressive strength of ground granulated blast furnace slag concrete using artificial neural network. Adv Eng Softw 40(5):334–340. https://doi.org/10.1016/j.advengsoft.2008.05.005
Bílek V, Kalina L, Novotný R, Tkacz J, Pařízek L (2016) Some issues of shrinkage-reducing admixtures application in alkali-activated slag systems. Materials. https://doi.org/10.3390/ma9060462
Duxson P, Lukey GC, Separovic F, van Deventer JSJ (2005) Effect of alkali cations on aluminum incorporation in geopolymeric gels. Ind Eng Chem Res 44(4):832–839. https://doi.org/10.1021/ie0494216
Fernández-Jiménez A, Palomo JG, Puertas F (1999) Alkali-activated slag mortars mechanical strength behaviour. Cem Concr Res 29:1313–1321. https://doi.org/10.1016/S0008-8846(99)00154-4
Gasca-Tirado JR et al (2019) Porous geopolymer as a possible template for a phase change material. Mater Chem Phys 236:121715. https://doi.org/10.1016/j.matchemphys.2019.121785
Mabille (2013) Fragmentation of emulsions in a simple shear stress flow. PhD, University of Bordeaux, France
Marcus Y (2010) Surface tension of aqueous electrolytes and ions. J Chem Eng Data 55(9):3641–3644. https://doi.org/10.1021/je1002175
Ramsden W (1904) Separation of solids in the surface-layers of solutions and ‘suspensions’ (observations on surface-membranes, bubbles, emulsions, and mechanical coagulation).—preliminary account. Proc R Soc London 72:477–48
Rouquerol J, Rouquerol F, Llewellyn P, Maurin G, Sing KS (2014) Adsorption by powders and porous solids: principles, methodology and applications. Academic Press, London
Stephant S (2015) Etude de l’influence de l’hydratation de laitiers sur les propriétés de transfert gazeux dans les matériaux cimentaires. PhD thesis (in French), Bourgogne University
Tadros TF (2013) Emulsion formation, stability, and rheology. In: Tadros TF (ed) Emulsion formation and stability. Wiley, Germany, pp 1–75
Thommes M, Kaneko K, Neimark AV, Olivier JP, Reinoso F-R, Rouquerol J, Sing KSW (2015) Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution. IUPAC Technical Report, DE GRUYTER
Yamaji A, Masuda F (2005) Improvements in graphical representation of fabric data, showing the influence of aspect ratios of grains on their orientations. J Sediment Res 75(3):514–519. https://doi.org/10.2110/jsr.2005.040
Acknowledgements
This project has received technical support from: Renaud Podor working at the Institut de Chimie Séparative de Marcoule, UMR 5257 CEA-CNRS-UM2-ENSCM, Site de Marcoule, BP17171, F-30207 Bagnols sur Cèze Cedex, France for Environmental Scanning Electron Microscopy observations. Bertrand Revel working at the University of Lille for solid-state NMR measurements.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Reeb, C., Davy, C.A., De Campos, M. et al. How are alkali-activated materials impacted by incorporating low viscosity organic liquids?. Mater Struct 56, 11 (2023). https://doi.org/10.1617/s11527-022-02089-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1617/s11527-022-02089-2