Abstract
The application of chemical analysis on bituminous materials has increased drastically over the past decades. One of the most common spectroscopic methods used in the field of research is Attenuated Total Reflection (ATR) Fourier Transform Infrared (FTIR) spectroscopy. Since ATR-FTIR is a surface sensitive method, sample or specimen handling of a complex material like bitumen prior to its analysis needs to be considered, especially for people new to the field or analysis technique. This study looks at the impact of heating time and temperature as well as storage time and conditions on the oxidation of the bituminous specimen. Four binders from the same crude oil source but different specification classes (unmodified and styrene–butadiene–styrene polymer modified) and two binders from different crude oil sources were investigated. The results show that heating small quantities of bitumen at 180 °C for up to 30 min has little impact on the formation of oxidized species, when proper thermal monitoring is conducted. Special cases where oxidation does occur are reported in detail. Furthermore, strong oxidation is induced by day light, when bitumen is stored behind glass with no UV radiation present, which can reach short-term ageing level within 1 h. Thus, heating bitumen at 180 °C for 5–10 min followed by storage in the dark, climatized room and measured within one hour after preparation is recommended. These results should act as recommendation for future specimen handling prior to FTIR spectroscopic analysis to ensure unbiased and comparable measurements.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Bitumen is a product widely used as a binder in asphalt pavement or as a component in roofing membranes. Since it derives from the crude oil refinement process, it mainly consists of millions of different hydrocarbon molecules which exhibit a unique temperature dependent, viscoelastic behavior [1]. This molecular diversity makes it very complex to characterize as it is simply not possible to describe every single molecule within bitumen. Therefore, chemical analysis that provides a certain overview of the materials composition or molecular groups is desired. In addition, this method should be able to track changes which occur during the materials lifetime, which is usually linked to oxidation.
One, if not the most promising candidates to fill this role is Fourier Transform Infrared (FTIR) spectroscopy. Since there are many molecules in bitumen that are infrared active, meaning that upon absorption of infrared light these molecules start to vibrate or rotate, it is seen to be a fitting analysis technique. Furthermore, the intake of oxygen during ageing leads to the formation of functional groups like ketones or sulfoxides, which are all infrared active. The ability to show infrared activity depends on whether the molecules change their dipole moment upon absorption of energy. This provides a characteristic band for each infrared active molecule which can be accessed and reused to characterize or fingerprint unknown materials or substances.
The application of FITR spectroscopy, especially for bitumen has increased drastically during the last decades. One reason for this is the implantation of attenuated total reflection (ATR)-FTIR, where the specimen is applied onto a solid crystal and measured. Before ATR, measurements in transmission mode were standard procedure [2,3,4]. Since bitumen in its solid state is not transparent, it had to be dissolved and applied onto a suitable substrate like a KBr, CsI or NaCl window [5]. This resulted in longer specimen preparation and waiting times for solvent evaporation that reduces the methods applicability and simplicity. A measurement on an ATR-FTIR spectrometer is much simpler and faster, as the solid bituminous material can directly be applied onto the ATR crystal and measured. This crystal, typically a diamond, germanium or zinc selenide, is usually sintered into a metal plate which makes it overall robust and resistant to external stress.
However, even when measuring bitumen with an ATR-FTIR spectrometer many parameters need to considered to achieve good repeatability and reproducibility, which are being touched on by researcher and groups during the last decades. Hofko et al. [6] have performed measurements on an unmodified and polymer modified bitumen in regards to achieving a sufficient repeatability and testing the sensitivity of ATR-FTIR spectroscopy. They tested various data treatment options including spectral normalization followed by either baseline or tangential integration. Other quantification methods have also been proposed by researchers from the BRRC [7] or LCPC [8], or participants from the MURE project [9], which suggest the utilization of a valley-to-valley or deconvolution method for quantification of IR bands. While this integration can indeed provide a good outcome for sulfoxide and ketone formation, it neglects an overall increase in the fingerprint region which is linked to the polarity shift in bitumen occurring during ageing [10]. Another, more advanced spectral data analysis methods is proposed by Weigel and Stephan [11], which makes use of standard normal variant (SNV) transformation and multivariance analysis to detect or predict the properties of a binder in regards to its crude oil origin.
Beside the engagement by single researchers or small task groups in respective countries, FTIR spectroscopy has been touched on by various international research committees. The RILEM TC 206 ATB (Advanced in Interlaboratory Testing and Evaluation of Bituminous Materials) have used FTIR spectroscopy and proposed an approach for spectral interpretation [12]. The TC 252 has tested laboratory aged bitumen at different temperatures with FTIR spectroscopy [13]. Furthermore, the task group 1 of RILEM TC 272 PIM has looked into a similar round robin test with more advanced data treatment [14]. Detailed results are on their way towards journal publication. Task group 1 of the RILEM TC 295-FBB is currently looking to bring FTIR spectroscopy on a way towards pre-standardization, where over 25 laboratories from all across the world are participating and building up on the previously gathered knowledge. All in all, this shows how many possibilities for FTIR spectroscopy and data treatment are available up to date. Ultimately, there will most likely be various approaches on how to best address certain aspects of FTIR spectroscopy by different data treatment approaches which tackle questions like ageing, additives or crude oil origin.
All the previously displayed literature has mostly worked in different data treatment approaches. However, a factor that has not been addressed often is handling bitumen prior to its spectral analysis which plays a crucial role in FTIR spectroscopy. A reason for this is the fact that infrared light in the range of 25.000–2.500 nm (4000–400 cm−1) is used to analyze the material. This light only penetrates up to a couple of microns into the material, which means that this method investigates a small portion of molecules at the materials surface [15]. Thus, to obtain a meaningful result, proper representation of the materials molecular composition is necessary, meaning that homogeneity becomes a crucial factor for spectroscopic analysis. To achieve a good homogenization, continuous stirring during specimen preparation is recommended, especially for small binder quantities. Hence, in order to ensure that the material can be homogenized, it needs to be in a liquid like state, thus higher temperatures are required.
This leads to the second important parameter that needs to be addressed during specimen preparation: the heating temperature and time. Bitumen is typically heated up to higher temperatures (150–200 °C) during its processing cycle. Since it is an organic material, its susceptibility to oxidation is given by its nature. Therefore, the necessity arises to define a sufficient temperature and heating time span where limited oxidation is occurring while the material is brought into a well workable and homogenized state.
The third and last parameter address in this study is the storage time and conditions of the specimen. While for most materials it does not matter how they are stored in between specimen preparation and the measurement, such small specimens of bitumen do in fact show significant changes on the surface when being stored at the wrong conditions. This was highlighted by a recent study where bitumen specimen showed significant oxidation when stored in daylight under a glass lid in a climatized laboratory [16]. While the bulk of the materials is not affected as much, the ageing level on the surface can reach even short- and long-term ageing level after being stored under a glass dish for 3 or 6 days respectively. Hence, some special care needs to be taken when storing specimen for ATR-FTIR spectroscopy.
The goal of this study is to address various parameters that need to be considered for preparing bitumen for ATR-FTIR spectroscopy. This includes the impact of heating time and temperature as well as different storage times and conditions. Two commonly available heating devices, a ventilated oven and heating plate, are used to heat up small bitumen quantities (5 g) for 5–30 min at 180 °C. Various binders from the same crude oil source but different specification classes (unmodified and styrene–butadiene–styrene (SBS) modified), ageing states as well as different crude oil sources were tested. Furthermore, a more detailed investigation on the storage time and conditions was conducted. The main question arising here were how long can a bituminous material be heated and stored before showing significant signs of oxidation and whether the binders viscosity has a major impact on the oxidation rate. Finally, something that should be kept in mind that while many laboratories have already acquired an FTIR spectrometer and developed their routine, there are other people who new to the field of research and are not familiar with either working with binder or ATR-FTIR spectroscopy. Thus, this study intents to provide recommendations on how to handle bituminous materials prior to their analysis on the ATR-FTIR spectrometer.
2 Materials and methods
2.1 Materials
The bitumen used in this study were four binders from the same crude oil source with the following specification classes:
-
Binder A—unmodified 50/70 penetration graded bitumen (PG 64–28)
-
Binder B—unmodified 70/100 penetration graded bitumen (PG 58–28)
-
Binder C—unmodified 160/220 penetration graded bitumen (PG 46–34)
-
Binder D—SBS modified Binder 45/80–65 (PG 82–28)—(Binder B was the base bitumen)
Furthermore, two additional 70/100 specification graded binders from different crude oil sources were also investigated, which will be labeled as follows:
-
Binder E (PG 58–28)
-
Binder F (PG 64–28)
All binders were investigated in the unaged state, as their changes caused by oxidation would be most notable. Merely binder B (a binder typically used in road pavements in the central part of Europe) was analyzed in laboratory short- and long-term aged stated to determine the extent of oxidation that occurs during laboratory short- and long-term ageing and compare it to the impact of heating time and effect of storage. The laboratory short-term ageing, a Rolling Thin Film Oven Test (RTFOT) was performed according to the EN 12,607-1 [17] and for the laboratory long-term ageing, the Pressure Ageing Vessel Test (PAV) according to the EN 14,679 [18].
2.2 Specimen preparation
As this study was investigating five unmodified and one SBS modified binder, a heating temperature of 180 °C was chosen, which ensures that even the PmB can be brought into a well workable state. Two common heating devices, a heating plate (ARGO LAB M2-A) and a ventilated oven (Binder FD 23), which were both set to 180 °C, were used to heat up the binders. For specimen preparation, 5 g of the respective binder was transferred into a metal can (d: 40 mm, h: 13 mm) and either placed on a:
-
heating plate (metal can without a lid),
-
ventilated oven (metal can with or without a lid)
as displayed schematically on the left side in Fig. 1.
While placed on the heating plate, the binder was stirred continuously with a thermometer. This ensures sufficient homogenization during the heating phase and reduces the risk of inhomogeneity when preparing the specimens after the heating phase. Furthermore, it allows thermal monitoring, which ensures that the binder does not exceed a certain temperature. Nevertheless, something to consider is that higher oxidation could be induced due to the continuous stirring, which will be tested throughout this study.
When placed in the oven on the other hand, the bitumen could not be stirred. However, higher temperatures around the binders could be achieved, as the air atmosphere is hotter compared to the heating plate. Especially without a lid on the can, hot air is blowing directly over the bitumen film, which can lead to a strongly oxidized surface (which will be shown in the results). To tackle this problem further tests with a closed metal can in the ventilated oven were conducted throughout this study, which can reduce oxidation, as later results will show. Disadvantages of a closed metal can are also addressed throughout the results.
Once the binder was heated up for the respective heating time, the thermometer was used to once again stir/homogenize the binder one last time and apply a droplet onto a sufficient substrate (silicone foil or paper slip). For each binder, 4 droplets were taken from the metal can after 5, 10, 20 and 30 min of heating and prepared according to the right side of Fig. 1. In this study, a silicone foil was used as a substrate, as it is reusable for many measurements and does not need to be thrown away, as the bitumen does not stick on it. However, alternatively a slip of paper can also be used as a substrate.
The four specimens per binder and heating time were placed in a crystallization dish, covered with a metal lid and left to cool down to room temperature for a couple of minutes. It is important to note that the specimens were measured within 60 min after preparation.
2.2.1 Variation of heating temperature
In addition to the heating time tests at 180 °C another small experimental study using a more extreme temperature of 240 °C was conducted. It is common knowledge that bitumen should not be heated above 200 °C as it can start smoking which is linked to an evaporation of volatile compounds. However, it is unclear if any significant oxidation occurs when the material starts smoking and whether the loss of these volatile compounds can be detected in FTIR spectra. To keep this study as compact as possible merely binder B was selected to investigate this question. Specimen preparation was performed as mentioned previously (5, 10, 20 and 30 min in the ventilated oven and heating plate and subsequent preparation of four specimens per heating time).
2.2.2 Variation in storage time and conditions
Beside the variation of the heating time and temperature, storage time and conditions were investigated, as they seem to show a significant effect on the oxidation level of bitumen, as described in the introduction. Since previous literature looked at binders within the same specification class but from different crude oil source, this study intended to test whether any differences in oxidation can be linked to the binder’s specification class (e.g. do softer binder take up oxygen faster). Thus, only Binder A–D were tested.
The specimens prepared for the storage study were heated on a heating plate for 5 min at 180 °C with continuous stirring and thermal monitoring before being applied onto the silicone foil, placed in a crystallization dish and covered with a metal lid (dark) or glass lid (light) to prevent contamination from dust (see right bottom side of Fig. 1). Four specimens for each day and storage condition (light or dark) were prepared and measured after 0, 1, 2, 3, 6, 9, 14 and 20 days as well as 1, 2, 3, 4, 5, 24 and 48 h after preparation. The reason for the second (hours) storage study will be elaborated in the results.
2.3 Analysis method
2.3.1 ATR-FTIR spectrometer and parameters
A Bruker Alpha II FTIR spectrometer equipped with an attenuated total reflection (ATR) unit containing a diamond crystal and a DTGS detector was used in this study. Spectra were recorded within a wavenumber range of 4000–680 cm−1, a resolution of 4 cm−1 and 24 scans. Before a bitumen specimen was applied onto the crystal, a background spectrum of the empty, clean ATR crystal was recorded. Once the background was recorded, the specimen was applied onto the crystal within a time window of 1 min. Longer waiting time can lead to a shift in the baseline, as the background can change. Each specimen was scanned with four repetition, resulting in four spectra per specimen. Since 4 specimens per binder and heating time were prepared a total of 16 spectra were recorded. After the measurement, a suitable, nontoxic bitumen solvent (limonene) was used to clean the diamond crystal. Since the limonene is an oily liquid, a fast evaporating alcohol (isopropanol) was used to remove any remaining greasy film.
2.3.2 Spectral data evaluation and plotting
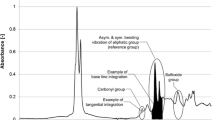
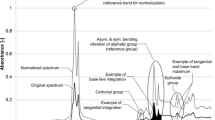
In order to keep the data evaluation process as simple as possible, the recorded spectra were directly processed in the attached software OPUS which is linked to the Bruker device. In the first step, all spectra were normalized to the band with the highest intensity in the bitumen spectrum, the aliphatic band at 2920 cm−1. A min–max normalization in the range of 3200–2800 cm−1 was carried out, which reduces the impact of any error coming from the ATR crystal as it has a characteristic signal in the range between 2300 and 1800 cm−1, which often represents the lowest intensity area in the original spectrum.
After normalization a full base line integration was performed on the three functional groups shown below:
-
Carbonyls (AICO): 1660–1800 cm−1
-
Sulfoxides (AISO): 1079–984 cm−1
-
Reference aliphatic band (AICH3): 1525–1350 cm−1
The received values were then used to calculate the ageing index (AIFTIR) according to Eq. (1).
The 16 aging indices per binder (at a defined heating time or storage time and condition) were statistically evaluated by mean and standard deviation and are shown in form of bar diagrams throughout the results.
From the 16 spectra recorded per binder, one spectrum was selected for plotting using OriginPro2021b. The spectra show only the finger print region (1800–680 cm−1), as it shows the most bands and significant changes in the bitumen spectra. It should be noted that while the min–max normalization was set in between the ranges of 3200–2800, this had no negative effect on the fingerprint region. A good accordance between raw data and normalization was found for all data.
However, it should be kept in mind that there are many ways to do spectral data evaluation. Differences in the normalization or integration method can lead to different results. Since thermal oxidation of bitumen is detected by an increase in carbonyls and sulfoxides as well as a slight intensity increase in the fingerprint region this rather simple data evaluation was deemed sufficient. The normalization process is not impacted by the scattering noise of the ATR crystal and also not by any potential changes in the fingerprint region, while still maintaining a good repeatability, as shown in the results. Additionally, it enables other researches to try out the herein proposed data analysis method without putting a lot of effort in it (since spectral analysis is usually performed by the software attached to the spectrometer). Nonetheless, various data treatment approaches will be tackled in the near future to determine which provide the best outcome for answering the respective questions asked.
3 Results and interpretation
3.1 Variation of heating time
3.1.1 Comparison of different specification classes
Binder A – D were all originating from the same non-naphthenic crude oil source and refinery procedure, which leads to similar FTIR spectra, as their molecular diversity is rather low compared to other crude oil sources (e.g. the abundance of naphthenic acids that show an initial ketone signal at 1700 cm−1). Therefore, only the normalized spectra of binder A (50/70) are depicted as an example in Fig. 2. Here, one can see that heating 5 g of a bituminous material on a heating plate at 180 °C (left side in Fig. 2) does not induce significant oxidation. Even when the sample was stirred continuously with a thermometer during the entire heating duration, merely a slight formation of sulfoxides at 1030 cm−1 or increase in the respective region due to the increase in polarity is observable. The significance of this sulfoxide formation will be touched on when looking at the deviation in the aging indices below. The right side of Fig. 2 shows the spectra of binder A (50/70) that was heated in the open metal can in a ventilated oven. Compared to the heating plate, oxidation is induced with increasing heating time, even when the sample was not stirred during the heating process. This is amplified by the green boxes on the righthand side in Fig. 2, which show a slight increase in ketones at 1700 cm−1, aromatics at 1600 cm−1 and sulfoxides at 1030 cm−1. This indicates that the temperature of the air that is in contact with the bitumen surface plays a major role in the oxidation kinetics. Even when the binder itself is at 170–180 °C, no significant oxygen uptake is seen on the heating plate, as the air atmosphere is at much lower temperatures. However, when hot air is in direct contact with the bitumen surface, the oxygen uptake is significantly increased. This could be explained by the higher reactivity of the oxygen at elevated temperatures. To simply these trends for the other binders investigated, the respective ageing indices (a combination of carbonyls and sulfoxides) will be shown below.
Looking at the ageing indices of binder A–D, which is shown Fig. 3, the difference between heating plate and oven becomes obvious. The heating plate only induces little (binder C and D) to no oxidation (binder A and B) after 30 min of heating. This oxidation is mainly attributed to the formation of sulfoxides, which can be explained by comparing the electronic configuration of the two elements involved in the oxidation: sulphur ([Ne] 3s2 3p4) and carbon ([He] 2s2 2p2). Since a sulphur atom is larger, their outer electrons (3p4) are further away from the atom core compared to the outer electrons of carbon (2p2). Thus, the ionization energy of sulphur (10.4 eV) is lower than of carbon (11.3 eV) [19]. It should be kept in mind these two elements are incorporated in a complex molecular matrix, which can alter their respective ionization energies and reactivity. Nonetheless, this can explain why sulfoxides, even at light oxidation conditions, are formed first. The heating in the ventilated oven on the other hand leads to a significant formation of both carbonyls and sulfoxides with increasing heating time, as shown on the right side in Fig. 2. Furthermore, a significant increase in the standard deviation can be seen for longer heating times in the ventilated oven. Thus, longer heating times make it more difficult to reflect the actual ageing level of a binder and achieve a good repeatability. An interesting observation can be made when comparing the PmB (binder D) to its unmodified counterpart (binder B), which shows lower oxidation susceptibility for longer heating times in the ventilated oven. This could indicate that a PmB, which might exhibit some pre-oxidation from the manufacturing, can endure more thermal stress.
Comparing the ageing indices of all four binders that originate from the same crude oil source, a different starting level can be observed with the softest binder exhibiting the lowest ageing indices. An explanation can be given by the full baseline integration method, as the overall intensity level of the binder in the fingerprint region is lower for softer binders, resulting in a lower ageing index. This can be linked to the binder’s polarity gradient, where the softer binder C contains less asphaltenes compared to the harder binders A and B [20]. Again, the hypothesis can be made that softer binders (e.g. binder C) oxidize faster than harder (binder A and B) or polymer modified binders (binder D). To confirm this assumption, the ageing index after 5 and 30 min are compared in the form of Δ AIFTIR (5–30 min) and are given in Table 1. Even though these tables partially replicate data shown in Fig. 3, the comparison between 5 and 30 min as well as the exact values of the respective deviations were deemed necessary for further interpretation when defining a threshold for maximum deivation.
Concluding the data from Table 1, storing bitumen in an open metal can inside a ventilated oven can cause significant increase in the oxidation during 30 min of heating (Δ AIFTIR (5–30 min) values of 0.012–0.031) as well as an increase in the standard deviation (0.004–0.013). Heating the sample on the heating plate induces much less oxidation, but still can reach AIFTIR (5–30 min) values of 0.001–0.012 a after 30 min of heating, while maintaining a low standard deviation of 0.001–0.002. Thus, a heating time of 5–10 min (for quantities of 5 g) are recommended. This ensures that the material can be brought into a well workable and homogenized state which shows little oxidation and a small standard deviation. However, in order to determine the significance of such a deviation of the binder’s oxidation level in regards to heating time of up to 30 min, a comparison to the short- and long-term ageing level was deemed useful.
3.1.2 Comparing different ageing states
Hence, a laboratory short- and long-term aged binder was also exposed to the same thermal treatment (5, 10, 20 and 30 min) in the oven and heating plate and compared to the unaged binder. In order to keep this study compact yet informative, merely binder B was selected, as it was in the middle of the penetration grade range, commonly used in central Europe and was also the base binder of the PmB shown previously.
Figure 4 shows the resulting FTIR ageing indices of the unaged, RTFOT and RTFOT + PAV aged binder B. When comparing the ageing level of the sample prepared on the heating plate (left side) no significant increase in oxidation can be seen after 30 min, as the Δ AIFTIR (5–30 min) for the RTOFT (0.003) and PAV (0.003) aged binder stay below the recommended value of 0.005 and exhibit a small standard deviation (0.001–0.003). Heating the sample in the ventilated oven surprisingly only induces oxidation at the unaged level (as discussed previously). Once the binders have reached short- or even long-term ageing state, a slight increase of carbonyls and sulfoxides is detectable as the Δ AIFTIR (5–30 min) values for the RTOFT (0.006) and PAV (0.004) aged binders are close to the recommended value of 0.005. Furthermore, their respective standard deviations are in a range of 0.001–0.003, which is below the recommended value of 0.005. The meaningfulness of these results will be discussed later.
3.1.3 Comparison of open and closed metal container in the oven
Since storing an open bitumen can in the ventilated oven can lead to significant oxidation with increasing heating times, a small study tested whether a closed can in the oven can produce a smaller degree of oxidation. Once again, binder B was used, as it also had shown significant oxidation when an open can was placed in the ventilated oven. Furthermore, this mainly seems to affect unmodified and unaged binders, as the previous results shows that an unmodified short- and long-term aged binder or an unaged PmB did not oxidize as strongly as their respective unaged and unmodified counterparts.
Figure 5 shows the resulting ageing indices of binder B heated in the open can on the heating plate, closed and open metal can in the ventilated oven. Looking at the right-hand side of the graph, it becomes obvious that when covering the metal can with a lid, the degree of oxidation, even after 30 min in the ventilated oven, are minimal compared to an open can. This is also confirmed by a ΔAIFTIR (5–30 min) value of 0.005 as well as a standard deviation of 0.004, which are both within the recommended limit of 0.005. The only disadvantage of a closed metal can in a hot oven arises when the lid of the metal can need to be removed quickly from the can in order to prepare the samples while the binder is hot. This can become tricky with small metal cans as thick protection gear needs to be used when touching hot metal surfaces.
3.1.4 Comparison of different crude oil sources
To broaden the significance of the results, two additional 70/100 penetration graded binders from different crude oil sources were exposed to the same test program as the previous binders. Since the storage in the ventilated oven without a lid induced significant oxidation, the oven heating was performed with a metal lid on and compared to the heating plate.
The resulting ageing indices of binder E and F are shown in Fig. 6, which indicate that when placed on a heating plate under continuous stirring, a better repeatability could be maintained as the standard deviation ranges between 0.001 and 0.005. However, after 30 min of heating, binder E and F exhibit a Δ AIFTIR (5–30 min) value of 0.005 and 0.008 respectively. This indicates that after long heating times the recommended oxidation threshold limit of 0.005 is reached or even surpassed. The samples heated in the ventilated oven (with a closed metal can) on the other hand show a higher standard deviation range (0.001–0.011) as well as higher ΔAIFTIR (5–30 min) value for binder E (0.012) but lower for binder F (0.004). Thus, while binder E showed higher oxidation in the ventilated oven, binder F formed more oxidizes species on the heating plate. Overall, the heating plate produces lower standard deviations, compared to the ventilated oven, which can be assigned to the lack of continuous stirring in the ventilated oven.
3.2 Variation of heating temperature
The previous results show that a heating temperature of 180 °C can, when neglecting certain parameters, induce oxidation. However, this was only the case for open can stored in a ventilated oven and long heating times, which is not recommended for heating small sample quantities of bitumen. Other than that, 180 °C did not induce significant oxidation within 5–10 (or even 30) minutes of heating using the oven or the heating plate and was deemed suitable as it brings many binders into a workable state. Thus, lower temperatures were not interesting to investigate, since these cannot be applicable for polymer modified binders or strongly aged binders. Once again, this study was only performed on binder B, as it was the most used binder in this study. Figure 7 compares the resulting ageing indices of binder B heated at 180 °C (left) and 240 °C (right).
Here, one can see that while higher temperature does induce a slight increase in oxidation over time, resulting in a ΔAIFTIR´(5–30 min) value of 0.008 (heating plate) and 0.009 (closed can in ventilated oven) with an overall small standard deviation (0.001–0.002). Thus, only a slight increase in oxidation occurs during the heating time of 30 min. The meaning of these results will further be elaborated in the discussion.
3.3 Variation in storage time and conditions
As previously mentioned in the introduction, the impact of storing bitumen specimens in the dark or under light has a significant impact on the oxidation level on the surface [16]. Since these specimens were always covered with a glass that absorbs UV light, it indicates that visible light causes major oxidation on the bitumen surface. However, the study by Mirwald et al. [16] only looked at two 70/100 penetration graded binder from different crude oil sources which showed both a similar increase in carbonyls and sulfoxides with rising storage time. Furthermore, it only looked at larger time windows (days). This study tries to solve the questions whether the specification class (which is linked to differences in physical parameters like viscosity) has an impact on the oxidation rate on the surface. It could be assumed that e.g. softer materials with a higher molecular movement can incorporate more oxygen which would lead to a higher formation rate of carbonyls and sulfoxides. Furthermore, beside a larger time span of up to 20 days, a shorter time span looks at the formation of carbonyls and sulfoxides within the first 5 h after specimen preparation.
Figure 8 shows the spectra of binder B (unmodified 70/10) and D (PmB) stored in the light (left) and in the dark (right). On the left side, both binders stored in the light show the previously reported increase in ketones (1700 cm−1), aromatics (1600 cm−1) and sulfoxides (1030 cm−1) as well as overall increase in the region between 1350 and 900 cm−1 (and even further down to 680 cm−1). This increase in the finger print region can be linked to an overall increase in the polarity of the material [20]. Upon longer storage in the light, a small shoulder appears at 1760 cm−1 which could be assigned to other oxidation products like esters (e.g. cyclic esters) that exhibit a C=O stretching band in this region [21]. A second shoulder at 1110 cm−1, which can be assigned to the C-O vibration of esters further indicates or confirms their presence. The band at 1260 cm−1, an increase in the region around 1100 cm−1 as well as an increase at 810 cm−1 was previously assumed to be linked to the presence of organic sulphate esters (ROSO3−) [16, 21, 22]. Up to date no further chemical analysis has been conducted to identify them. While the overall oxidation in the material is drastically high, no deterioration or degradation of the polymer becomes visible for binder D, as the butadiene band at 960 cm−1 and the styrene band at 690 cm−1 remain after 20 days of storage. This indicates that the oxidation caused by visible light is only affecting the bitumen component, not the polymer. This behaviour is completely different from the thermal behaviour, where the PmB as a whole showed lower oxidation susceptibility to thermal stress. This highlights the difference between thermal and photolytic oxidation potentials, which will be addressed in future research.
Looking at the right side of Fig. 8, no significant change can be seen when the specimens are stored in the dark. A slight formation of sulfoxides at 1030 cm−1 coupled with an increase in the surrounding fingerprint region as well as the appearance of the bands assigned to the organic sulphate esters (ROSO3−) can be seen with increased storage time. This sulfoxide formation at mild conditions has been addressed previously.
The resulting ageing indices of Binder A–D, which were stored in the light and dark are shown in Fig. 9. A similar trend in increasing level of oxidation with storage time can be seen for all four binders, independent of the specification class or modification (with binder D being a PmB). By looking closer at all four graphs it becomes obvious that when specimens are stored in the light for one day, they surpass the ageing level of a short-term aged (RTFOT) binders. When stored in the dark, this level is only reached after 6–10 days. However, this is just due to the formation of sulfoxides, possible sulphate esters, not carbonyls, which becomes obvious when looking at the spectra on the right-hand side of Fig. 8.
Looking at the ageing indices of the binders stored in light for 20 days, it can be seen that polymer modified binder (binder D) exhibits the highest ageing index (0.82). This is followed by the 50/70—binder A (0.79), the 160/220—binder D (0.77) and 70/100—binder B (0.73). However, as previously mentioned, it needs to be kept in mind that the binder initial ageing index (AIFTIR @ Day 0) are slightly different which can be linked to differences in their SARA fractions [10]. Therefore, in order to judge the oxidation behaviour of all four binders, a previously reported power law fit was applied, as shown in the red curve in Fig. 9, which is summarized in Table 2. Herein, the oxidation rate is given by the coefficient b (in a logarithmic relation to time). All four binders stored in the light show an oxidation rate between 0.297 and 0.310, which indicates that the oxidation rate is more dependent on the crude oil source compared to the specification class (from the same crude oil source). One could argue that the 50/70 (binder A) exhibits the lowest oxidation rate, which indicates that softer binders incorporate more oxygen due to the lower viscosity and higher molecular dynamics, the deviation is however little. However, since this is a purely empirical observation, more sophisticated fitting would be necessary to really come to a conclusion in this regard.
Looking at the values from the storage in the dark an average oxidation rate of 0.05–0.06 can be seen. This is significantly smaller than the oxidation rate when stored in light (0.31–0.30), which shows that storage in the dark can prevent significant oxidation on the surface. Furthermore, the only oxidation occurring is a slight formation of sulfoxides, no carbonyls are formed (see right hand side of Fig. 8).
Since all these binders have already surpassed the ageing level of a RTFOT aged binder after being stored in the light for one day, further storage in the light testing at shorter time intervals after preparation was conducted. The only difference between the first and the second study was, that they were conducted 3 months apart from each other (Feb. 2021 and May 2021). Figure 10 shows the resulting ageing indices of the four binder specimens stored for 0, 1, 2, 3, 4, 5, 24 and 48 h in the light. Surprisingly, even after one hour stored in the light (binder A, C and D), the RTFOT ageing level was reached, which highlights the importance of proper storage conditions. Merely binder B’s ageing index after 1 h of storage in the light is slightly below the RTFOT ageing level. Looking at the ageing index after 48 h, which is also displayed in Table 3, a similar trend than for the 20 days storage test program was seen as all three unmodified binders show similar oxidation rates, which is again given by the coefficient b (in a logarithmic relation to time) from the power law fit. Merely binder D (PmB) exhibits a slower oxidation rate. Looking at right bottom side of Fig. 10, one can see that the fitting curve of the PmB is significantly below the final ageing index after 48 h, which explains these results. However, once again it should be kept in mind that the fit is not entirely appropriate but a rough approximation that captures the oxidation rate to a certain extent.
Another very interesting observation can be made when comparing the ageing indices of 48 h in Fig. 10 to the 2 days ageing indices in Fig. 9, which resemble the same storage time. Even though their ageing duration was the same, the binders from the hour storage study show significantly more oxidation than the binders from the 20 days storage study. A possible explanation can be given by the fact that the storage days study was conducted in February 2021, the hours storage study in May 2021. Since the specimens were stored in a climatized laboratory, temperature of the surrounding air was not a concern. Another factor that differs substantially is the sunshine duration. Looking at values from a local centre of metrology and geodynamics for the respective months it can be found that February 2021 had significantly lower values of sunshine (108 h) compared to May 2021 (182 h).Footnote 1 This indicates that the amount of daylight in combination with differences in sunshine on the specimen surface leads to significantly more oxidation, which is caused by the energy from light in combination with oxygen from the atmosphere. Further investigation on the impact of visible light will be conducted in the future, to see how significant this factor can be when investigating the phenomenon of bitumen oxidation.
4 Discussion
The previously displayed results have shown how parameters like heating time, temperature and storage time and conditions can cause oxidation which is well detectable by the increase in oxidized species with the ATR-FTIR spectrometer. While the overall impact of heating time does only become relevant at extreme conditions and times (e.g. an open metal container in a ventilated oven at 180 °C or longer heating times), the impact of storage can be quite significant, depending on environmental conditions (sun shine duration and temperatures during storage).
Thus, in order to obtain a good repeatability with ATR-FTIR spectroscopy various specimen of a bituminous sample should be prepared and measured. The herein proposed number of four specimen, which is combined with four repeated measurements and yield a total of 16 spectra per binder, which is sufficient for spectral analysis and statistical significance. While the proposed data evaluation has its advantages and disadvantages, the overall quality of the raw data was considered, which should be the first step for evaluation of FTIR spectroscopic results. However, when following the simple data evaluation approach proposed (min–max normalization followed by full baseline integration), a statistical deviation in the resulting ageing indices should be kept as low as possible, as it is linked to the binders ageing and the materials homogeneity. Thus, the results indicate that a value below 0.005 is sufficient for resembling good repeatability when following the data evaluation by min–max integration and full baseline integration with the software OPUS.
While this threshold has its significance for heating times of unaged materials, it is reduced for aged binders, as their overall susceptibility to oxidation during the heating is little as the material is already in an aged state. A potential error could mainly occur from inhomogeneity. While this is common knowledge for handling the material for mechanical investigations, no differences can be assumed for spectroscopic analysis. Nonetheless, the heating times should be kept as short as possible throughout a specimen preparation routine to keep the potential for oxidation as limited as possible while maintaining a good homogeneity. In addition to the heating time (independent of binder or ageing state) the heating conditions (closed or open container) for small binder quantities around 5 g can have a significant impact on the oxidation state of an unaged material. While heated on a heating plate, continuous stirring with a thermometer provides not only thermal monitoring but also proper homogenization. Heating the binder in an oven, the metal container must be covered with a lid to prevent excessive oxidation near the surface. Since different binders have already been measured and compared with ATR-FTIR spectroscopy, the respective impact on different preparation times and heating parameters have not been reported to such detail. The data acquired will be further used and different data treatment procedures mentioned in the introduction [7,8,9, 11] will be applied. This will hopefully answer which evaluation method yields the best results in regards to the respective question.
While FTIR spectroscopy seems to be capable of answer many important questions regarding the ageing state of a binder, the results of the extremely high heating temperatures (240 °C) highlighted a potential disadvantage of FTIR spectroscopy, since it does not provide spectral information whether the binder has been excessively heated (spectra—not shown—but they do not exhibit differences). However, it needs to be kept in mind that this is very binder specific. While this binder also did not show drastic smoking even at 240 °C, others can start even below 200 °C. Thus, to validate this assumption, more samples would need to be tested at 240 °C. Furthermore, it could be interesting to investigate the rheological properties of such a highly heated binder and see whether changes are detectable when comparing 180 °C to 240 °C. This could provide an answer whether the smoking of the material causes significant changes on the mechanical level which are not detectable by FTIR spectroscopy. Nonetheless, it shows that temperature alone does not have a significant impact on the formation of the two oxidized species during the heating, when following the presented parameters.
Discussing and reflecting the results of the second part, the storage study, it can be said that specimen storage can have a significant impact on the resulting spectrum as surface oxidation triggered by light is detected rapidly. This provides further insight into the impact or sample storage reported earlier [16], especially in regards to different specification classes. While it appears to be more or less independent of specification class, the actual weather conditions (daylight and sun light duration) can change this oxidation rate significantly. Beside the overall strong formation of carbonyls and sulfoxides an overall intensity increase in the fingerprint region can be detected. The findings recommend that bituminous specimen should be stored in the dark immediately and measured within an hour after preparation to induce no further oxidation on the specimen surface which ensures to reflect the actual ageing state of the material investigated with ATR-FTIR spectroscopy. An overview of these concluding remarks can be found below.
5 Conclusion
This study shows results on how different heating times and temperatures as well as storage times and conditions can affect bituminous materials when they are analyzed with ATR-FTIR spectroscopy. Four binders from the same crude oil source and refinery (hard–soft and a PmB) as well as two additional 70/100 penetration graded binders were investigated in an unaged state and partly in the laboratory short- and long-term ageing state.
These findings recommend heating small quantities (~ 5 g) of bituminous binders (independent of specification class or modification) on a heating plate while stirring for 5 min at 180 °C. This ensure proper homogenization and ideal thermal monitoring which will yield a good repeatability and low standard deviation (threshold value of 0.005 considering min–max normalization at the main aliphatic band and full baseline integration with broad ranges). Alternatively, the binder can be heated in a closed metal can inside a ventilated oven for 5–10 min. Leaving the metal container open can induce significant oxidation after longer heating times and will reduce repeatability. While the standard temperature used in this study (180 °C) was sufficient to bring all binders into a well workable state, a rather extreme temperature (240 °C) did no show significant differences in the spectra. This suggests that ATR-FTIR spectroscopy is not capable to measure whether a bituminous material has been heated too much.
Sample storage after preparation is a crucial parameter, as surface oxidation can occur even after one hour and are independent of the binder specification class but more linked to external conditions like daylight and sunshine duration during storage. Thus, storage in the dark (cover with a metal lid) for a maximum of 1 h before measurement on the ATR-FTIR spectrometer is recommended.
While the investigations tried to cover as many preparation parameters as possible, it needs to be seen whether they applicable to other laboratories and binders. Furthermore, topics like data evaluation have not been dealt with in detail. Further evaluation of the data by different approaches are planned in the future. However, these recommendations should act as a basis for handling bituminous materials prior to their analysis and achieve a good repeatability as it becomes one if not the most established spectroscopic method in the field of road engineering.
Notes
The values for the average sunshine duration are taken from local centre of metrology and geodynamics from the following source: https://www.wien.gv.at/statistik/lebensraum/tabellen/luftsonne.html
References
Lesueur D (2009) The colloidal structure of bitumen: Consequences on the rheology and on the mechanisms of bitumen modification. Adv Coll Interface Sci 145(1–2):42–82
Lamontagne J (2001) Comparison by Fourier transform infrared (FTIR) spectroscopy of different ageing techniques: application to road bitumens. Fuel 80(4):483–488
Petersen JC et al (2002) Molecular interactions of asphalt. Tentative identification of 2-quinolones in asphalt and their interaction with carboxylic acids present. Analyt Chem 43(11):1491–1496
Petersen JC (1975) Quantitative method using differential infrared spectrometry for the determination of compound types absorbing in the carbonyl region in asphalts. Anal Chem 47(1):112–117
Weigel S, Stephan D (2018) Differentiation of bitumen according to the refinery and ageing state based on FTIR spectroscopy and multivariate analysis methods. Mater Struct 51(5):1–11
Hofko B et al (2017) Repeatability and sensitivity of FTIR ATR spectral analysis methods for bituminous binders. Mater Struct 50(3):1–15
Pierard, N. (2013). Bitumen analysis with FTIR spectrometry: Processing of FTIR spectra. BRRC.
Mouillet, V., Farcas, F., Battaglia, V., Besson, S., Petiteau, C., & Lecunff, F. (2010). Identification and quantification of bituminous binder's oxygenated species, Analysis by Fourier Transfrom Infrared Spectroscopy. In LCPC testing method 69 Technical report ME69 (Ed.). LCPC.
Dony A, et al (2016) MURE national project: FTIR spectroscopy study to assess ageing of asphalt mixtures. In: 6th Eurasphalt & Eurobitume congress. Prague, p 11.
Mirwald J et al (2020) Investigating bitumen long-term-ageing in the laboratory by spectroscopic analysis of the SARA fractions. Constr Build Mater 258:119577
Weigel S, Stephan D (2017) The prediction of bitumen properties based on FTIR and multivariate analysis methods. Fuel 208:655–661
Partl, M., Bahia, H., Canestrati, F., de la Roche, C., Di Benedetto, H., Piber, H., & Sybilski, D. (2013). Advances in interlaboratory testing and evaluation of bituminous materials (Vols. State of the Art report of the RILEM Technical Committee 206-ATB). Springer.
Hofko B et al (2018) FTIR spectral analysis of bituminous binders: reproducibility and impact of ageing temperature. Mater Struct 51(2):1–16
Porot L, et al (2020) Complex bituminous binders, are current test methods suitable for? In: ISBM. Lyon
Pipintakos G et al (2021) Exploring the oxidative mechanisms of bitumen after laboratory short- and long-term ageing. Constr Build Mater 289:123182
Mirwald J, et al (2020) Time and storage dependent effects of bitumen—comparison of surface and bulk. In: Proceedings of the RILEM international symposium on bituminous materials. Lyon
CEN, EN 12607-1 (2015) Bitumen and bituminous binders—determination of the resistance to hardening under the influence of heat and air—part 1: RTFOT method, Brussels
CEN, EN 14769 (2012) Bitumen and bituminous binders—accelerated long-term ageing conditioning by a Pressure Ageing Vessel (PAV), Brussels
Haynes WM (2015) CRC handbook of chemistry and physics, 95th edn. CRC Press, p 39
Mirwald J et al (2020) Understanding bitumen ageing by investigation of its polarity fractions. Constr Build Mater 250:118809
Larkin P (2011) Infrared and Raman spectroscopy. Principles and spectral interpretation. Elsevier, Waltham, pp 129–130
Chihara G (1960) Medical and biochemical application of infrared spectroscopy. V.: infrared absorption spectra of organic sulfate esters. (1). Chem Pharma Bull 8(11):988–994
Funding
The authors acknowledge TU Wien Bibliothek for financial support through its Open Access Funding Programme. The financial support by the Austrian Federal Ministry for Digital and Economic Affairs, the National Foundation for Research, Technology and Development and the Christian Doppler Research Association is gratefully acknowledged. Furthermore, the authors would also like to express their gratitude to the CD laboratories company partners BMI Group, OMV Downstream and Pittel + Brausewetter for their financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mirwald, J., Nura, D. & Hofko, B. Recommendations for handling bitumen prior to FTIR spectroscopy. Mater Struct 55, 26 (2022). https://doi.org/10.1617/s11527-022-01884-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1617/s11527-022-01884-1