Abstract
Reinforcement corrosion is major reason for concrete structures deterioration. Chlorides from external sources such as seawater and de-icing salts penetrate in the concrete and as soon as a critical threshold reaches the reinforcement level corrosion processes start. Therefore, the characterization of the chloride ingress resistance in form of the rapid chloride migration (RCM) coefficient, DRCM is crucial to classify concretes for given applications and to enable full probabilistic service life prediction. To measure DRCM of chloride-contaminated concrete, a rapid iodide migration test was developed using iodide as penetration ion and an iodine–starch reaction for penetration depth indication. This indicator mixture has the disadvantage that it is not applicable on sulfide containing concretes such as ground granulated blast furnace slag concretes. In this paper, the reason for the unsuitability of this indicator is examined and alternative oxidation agents are found and validated to overcome this problem. The new indicator mixtures with hydrogen peroxide (H2O2) or/and potassium persulfate (K2S2O8) as oxidation agents are not only insensitive to sulfide contamination but are also applicable to common concrete compositions and could replace the existing indicator universally.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The major reason for concrete structures to deteriorate is the chloride-induced reinforcement corrosion, for instance when the structure is located in marine environment or exposed to de-icing salts. The durability design of concrete structures is slowly moving from prescriptive to performance-based specifications. Consequently, suitable and reproducible test methods are required to characterize the chloride transport in concrete. These test methods should be usable for performance specifications and quality control. However, durability depends on deterioration processes, which need years to develop. Performance specifications require short term tests, lasting not more than some weeks, including preconditioning of the specimens. Otherwise, the approach is not practical nor acceptable for conformity control purposes, given the current pace of concrete construction [1].
Besides quality control purposes full-probabilistic service live prediction [2] with regard to corrosion initiation due to chlorides demands as well an input parameter which describes the chloride transport in concrete.
The diffusivity and the chloride-binding capacity of concrete dominate the resistance against chloride ingress into concrete. This material resistance describes the apparent chloride diffusion coefficient Dapp(t). The Dapp(t) can be determined by chloride profiles from field data and/or laboratory diffusion tests [3, 4]. These diffusion tests take several weeks while the determination of the rapid chloride migration (RCM) coefficient of concrete DRCM by RCM test is a suitable alternative [5]. RCM test accelerates the chloride ingress by applying an external voltage and the test takes only several hours or few days depending on the concrete resistivity and the resulting current [6,7,8,9,10]. Nevertheless, the RCM test does not reflect the chloride binding in the same degree as a diffusion test does [8]. On-site concrete differs from laboratory concrete due to workmanship, curing and environmental conditions. Therefore, drilled cores of the concrete cover from existing structures can be used for the RCM test to reflect the on-site conditions.
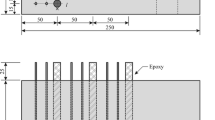
Figure 1 shows a schematic setup of the RCM test according to German Specification [11]. A concrete specimen of defined scales is placed in a migration cell creating a waterproof connection between cell and specimen. The cathode side is submerged in a 0.2 M KOH and 3 wt% NaCl solution while the anode is in contact with a 0.2 M KOH solution. Further setup details and the applicable currents and voltages can be found in the specific literature and standards [9, 11].
Schematic rapid chloride migration (RCM) test setup [11]
Drilled on-site concrete cores can already contain chlorides before the RCM test starts if a structure has been exposed to marine environment or de-icing salts. Furthermore, chlorides can be introduced to the concrete by chloride contaminated aggregates or water with chlorides. Since these internal chlorides migrate together with chlorides from the test solution, the RCM test is no longer applicable. The applied silver nitrate spray indicator [12] reacts with the migrated chlorides and the ingress is overestimated. This leads to a higher value for DRCM, thus, the full probabilistic service life model would indicate a much shorter time to corrosion initiation. Lay et al. [12] already published an alternative approach to estimate the chloride migration coefficient of chloride contaminated concrete: The rapid iodide migration (RIM) test. The test uses iodides instead of chloride ions as they have comparable migration behavior. The setup remains similar to the presented RCM test in Fig. 1, while the molar concentration of both test solutions is identical with 0.51 M (3.0 wt% sodium chloride for RCM test and 7.695 wt% sodium iodide for RIM test).
The indicator used for the RIM test is an water-based mixture of 3 wt% aqueous starch solution (400 mL/L), 50 wt% acetic acid (200 mL/L) and 1 M potassium iodate (KIO3) (400 mL/L). The oxidation of the penetrating iodide to iodine by iodate (reaction 1) is visible as a color change due to the blue iodine starch complex. Besides iodine, other byproducts of the reaction are sodium and potassium acetate (NaAc/KAc).
This indicator shows good contrast between iodide contaminated and iodide free zones, but only in concretes made with Portland cement. However, with increasing amount of GGBS cements (CEM III) used nowadays [13] no color change is observable. Although no iodine is present in the concrete mixture (reaction 2), the indicator reacts with sulfides present in the GGBS to the blue iodine starch complex covering the whole specimen. In this case, the iodine forming a blue iodine starch complex is produced by the KIO3 reduction itself, thus, a misinterpretation is possible. Besides iodine, other byproducts are also NaAc and KAc as well as sulfur (S) in the case of the sulfide containing solution.
Nowadays, the use of CEM III concrete is increasing due to energy efficiency, lower porosity, lower chloride migration coefficients [8] and higher resistance to alkali silica reaction (ASR) [14]. Frequently used CEM III B contains up to 80 wt% GGBS in which up to 2 wt% sulfide are present [15]. According to the pozzolanic reaction it contains less calcium hydroxide Ca(OH)2 than ordinary Portland cement. With regard of the increasing use of blended cements, this paper focuses on the development of an indicator to enable rapid iodide migration tests as well on GGBS and chloride contaminated GGBS concretes.
Two alternatives for KIO3 as oxidation agents for iodide are presented in this paper, which are not iodine containing but having the same oxidation power as IO3−: hydrogen peroxide (H2O2) is known to be a strong oxidation agent, it is a mass product with good availability and very riskless reaction products: water (H2O) and oxygen. Nevertheless, it is irritating and only stable in aqueous solution up to 30 wt%. Another alternative strong oxidation agent is potassium persulfate (K2S2O8) which is used as radical initiator for numerous polymerization types [16]. Subsequently, this paper describes the reaction of the currently used indicator with sulfides in vitro, on mortar prisms and on iodide/chloride contaminated cements. On the same systems, alternative indicators are developed and successfully tested.
2 Experimental
Figure 2 shows the experimental procedure: three different oxidation agents (one was the original KIO3 oxidation agent) were tested on samples contaminated with sulfides (A), chlorides (B), iodides (C), mixtures of the ions A–C (D) and a blind test in vitro and on concrete specimens.
2.1 Materials
All chemicals were purchased from Sigma-Aldrich (St. Louis, MO) or Merck (Darmstadt, Germany) and were used without further purification if not mentioned specifically. Water was purified by an arium pro ultrapure water system (Sartorius, Germany) prior to use. For pH measurement a ProLab 4000 pH meter (Schott Instruments, Germany) was used. All in vitro tests were performed in a saturated calcium hydroxide solution to simulate the alkaline pore solution of concrete. A 0.1 M sodium iodide (NaI) and a sodium sulfide (Na2S) solution represented the iodides and sulfides in concrete.
2.1.1 Indicator mixtures
In order to compare the new system with the original indicator [12], all concentrations and ratios were adopted and mixed as follows:
component 1:3 wt% aqueous starch solution and 50 wt% acetic acid (HAc) in a ratio of 3:1
Component 2 (oxidation agent) | Original system | |
|---|---|---|
0.21 M KIO3 solution | ||
Option A: | 30% aqueous H2O2 solution | |
Option B: | 0.21 M K2S2O8 solution | |
2.1.2 In vitro tests
1 mL 0.1 M NaI or Na2S or a mixture of both (1 mL 1/1 mixture) were mixed with saturated Ca(OH)2 solution, component 1 and different quantities of component 2 variations.
Titration experiments to observe the transition point of the H2O2 and K2S2O8 indicator system were prepared as follows: 3 mL 0.1 M NaI, 3 mL 30 wt% H2O2 or 0.21 M K2S2O8 respectively, 3 mL 50 wt% HAc and 9 mL starch solution were titrated to decoloration with 6 M sodium hydroxide (NaOH). Afterwards the colorless mixture was titrated back to blue with 50 wt% HAc. Acid and base were added dropwise into the vigorously stirring mixture while the pH value was monitored continuously.
2.2 Mortar tests
To transfer the in vitro system to a more realistic one, mortar prisms of 450 g GGBS containing cement CEM III/A 42, 5 N (Schwenk Mergelstetten, for detailed chemical composition check supplementary materials) 1350 g sand and 202.5 g water were prepared. Mixing regime followed DIN EN 196-1 [17] while the water-to-binder ratio was reduced to 0.45 due to better workability. NaI is added in various concentrations to the mixing water (0.21, 0.14, 0.07, 0.035, 0 mol/l NaI [12]) and the mortar specimens were stored for 17 days under tap water at 20 °C.
2.3 Concrete tests
To study the indicator systems under realistic conditions, migration tests with chloride and iodide were done according to Lay [12]: GGBS containing concrete was prepared with cement CEM III (same as for mortar) and 0/18 mm aggregates with water-to-binder ratio of 0.55. After 28 days RIM tests were conducted with a 0.51 M NaI solution, a 0.2 M potassium hydroxide (KOH) solution at the anode under 30 V for 24 h. In addition, a combined RCM/RIM test was conducted with 24 h RCM and 24 h RIM to penetrate chlorides and iodides into the concrete. Figure 3 shows a detailed description of the combined RCM/RIM test in accordance to [11].
To verify the applicability of the novel indicator also on ordinary Portland cement (OPC), CEM I concrete was prepared with CEM I 42.5 N (Schwenk Cement Mergelstetten) and 0/8 mm aggregates with water-to-binder ratio of 0.4. RIM/RCM specimens were prepared by drilling and sawing the specimens out of a concrete cube and a combined RCM/RIM test was conducted as described for CEM III specimen.
3 Results and discussion
3.1 In vitro tests
Table 1 summarizes the different variations of the in vitro tests with the corresponding observations. Various amounts of oxidation agent are tested while the maximal quantity is always 1 mL. One drop represents approximately the volume of 0.05 mL.
By adding 6 drops of the KIO3 solution as oxidation agent to the salt solutions, a color change from milky turbid to blue is observed independent of the type of salt (iodide or sulfide or mixed). The reaction is described earlier in Eq. 1. If more KIO3 is added a dark precipitate is observable. As mentioned in Eq. 2, in the case of sulfides (Table 1, line 3 and 4) a blue color is observed too, although no iodine is present.
Instead of KIO3, H2O2 works as an alternative oxidation agent in a NaI and NaI/Na2S solution if at least 5 drops 30 wt% aqueous H2O2 solution is added. No color change is observed if the indicator system is applied in a pure Na2S solution.
However, H2O2 is an economic alternative oxidation agent with water as a harmless reaction product. By using this oxidation agent in the adopted indicator system instead of KIO3 the net amount of formed iodide (reaction 3) decreases slightly compared to reaction 1. Nevertheless, a color change is also observed as the penetrating iodine is formed as an oxidation product. Together with the starch dissolved in the indicators’ spray solution the iodine can form the blue iodine–starch complex. If the solution contains sulfide no iodine is formed at all but elemental sulfur. Thus, the starch is not complexed and stays colorless as shown in reaction 4. The produced sulfur may cause turbidity as it is insoluble in water.
However, the question arises if the sulfide is oxidized to sulfur or to higher oxidation states like sulfate. In vitro tests with barium chloride yielded to no precipitate of barium sulfate when adding it to a test tube with the product of reaction (Eq. 4) which is also confirmed by literature [3].
K2S2O8 is tested as an additional oxidation agent variation. A 0.21 M K2S2O8 solution performs similar as H2O2 but takes more than 2 min to develop a clear blue color (see Fig. 4).
In principle, the redox reaction with K2S2O8 works analogous to H2O2 (reaction 5 and 6). However, no acetic acid is needed for this reaction based on Eq. 6. Slower reaction of K2S2O8 is due to sterical shielding of the reactive oxide–oxide bond compared to H2O2. Although K2S2O8 has a higher oxidation potential [18]. Besides iodine or sulfur, other byproducts of the reactions are sodium and potassium sulfate (Na2SO4/K2SO4).
A mixture of both (1/1) H2O2 and K2S2O8 solution as oxidation agent shows the same color changes as H2O2 and K2S2O8.
3.2 Mortar tests
Table 2 shows the different variations of the mortar tests with the corresponding observations. Mortar prisms with different concentrations of iodine are tested as well as reference samples without iodide.
In the case of H2O2 the blue color disappears after 1–3 min and the whole prism turns yellow starting from the edges (Fig. 5, line 6 Table 2). If the sample is sprayed again with H2O2-indicator mixture the blue color appears again. To analyze this phenomenon every constituent of the indicator was sprayed separately in a second turn. By using acetic acid the yellow color turns blue again.
Freshly broken surface of iodide-free prisms shows a yellow color by applying the H2O2 based indicator system, too (line 6, Table 2). To gain further insights of the color change from blue to colorless an in vitro titration experiment is performed. The experiment shows that with H2O2 the iodine starch complex is stable up to pH 8.6 and reversible colorful again from 7.0. After spraying the indicator on the surface, the surface dries which leads to the following two processes:
-
1.
The decomposition of remaining H2O2, which is not stable in the solid state.
-
2.
The surface realkalizes by diffusion of hydroxyl from deeper regions of the cement matrix.
Because of these effects, the behavior of iodine is dependent on reaction 7, shifting the chemical equilibrium to the right side and withdrawing the iodine out of the iodine–starch complex.
Acidification shifts the equilibrium back to the left side, producing iodine via a comproportionation reaction.
In case of the K2S2O8 based indicator system the color is more intensive blue. As in the previous in vitro tests, the color appears again on the prisms after few minutes. Nevertheless, no color change to yellow could be observed as in the case of H2O2. The repeated titration experiment shows that the iodine–starch complex is stable until pH 12.4 and from pH 12.1 colorful again. After drying, the K2S2O8 remains as a salt. Thus, as long as a stronger oxidation species than IO3− is present, the reaction 7 is hindered and reaction 5 remains decisive.
However, for small iodide concentrations the color is not as shiny and clear as for high iodide concentrations. Table 3 shows mortar prisms with several iodide concentrations in mixing water and the corresponding color after the application of the indicator.
As can be seen in Table 3, the new indicator systems has a lower detection limit for iodide. The color change boundary of the KIO3 based indicator corresponds to an iodide concentration of 0.07 mol/l. The new system is more sensitive since the boundary concentration is about 0.035 mol/l and therefore a new correlation factor between RCM and RIM needs to be calculated.
3.3 Concrete tests
To show the applicability of the indicator mixtures on a realistic system several concrete migration tests are executed. Basic indicator investigations are done on a CEM III concrete after a RIM test of 19 h. A combination of 24 h RCM followed by 24 h RIM is tested on a CEM III and a CEM I containing concrete, respectively (Fig. 3). The aim of the first step is to produce a concrete specimen with high chloride content. H2O2 and K2S2O8 based indicator systems are used similar to the original mortar tests. In the combined RCM/RIM test the chlorides migrate for 48 h while iodides migrating only 24 h (Fig. 3). Consequently, the chloride penetration depth, visualized with silver nitrate (AgNO3)/fluorescein shows a deeper migration front in the combined RCM/RIM test
The following Table 4 shows the different variations of the indicator tests with the corresponding observations.
By applying the existing KIO3 indicator spray test on GGBS containing concrete a blue color is visible on the whole surface. With the H2O2 indicator system a clear migration front is visible but it turns yellow after 5 min. However, a stable blue color is achieved after the second time spraying as shown in Fig. 6 and already discussed before. However, the yellow color of iodide free areas is an additional advantage of this indicator system since it creates a better contrast to blue on the dark surface of a GGBS containing concrete. The novel indicator systems are not affected by chloride contamination as seen in line 6, 7, 8, 10, 11 in Table 4. However, a K2S2O8 based indicators need more time to fully develop its color as well.
For the combined experiment of RCM and RIM, which should simulate the real on-site chloride contamination condition, the system was successfully tested. The new indicator is suitable to indicate the migration depth of iodide in sulfide containing concrete compositions.
To validate the new indicator systems on standard concrete mixtures, samples with ordinary Portland cement CEM I are used for the RIM test. Table 5 gives the overview of the indicator test variations with the corresponding observations.
However, for H2O2 the color front is visible after spraying twice while for K2S2O8 only spraying once was necessary, as it takes longer to yield to color change.
In the case of the more alkaline CEM I both indicator systems verified the applicability of the developed system. However, in the H2O2 system acetic acid need to be sprayed again to stabilize the color.
The main objective of this investigation is the determination of DRCM in chloride-contaminated concrete, as the full-probabilistic service live prediction requires DRCM as input parameter. Therefore, Lay calculated DRCM using measured DRIM data multiplied with a correlation factor \(\frac{{D_{\text{RCM}} }}{{D_{\text{RIM}} }}\) obtained from the comparison of both ion penetrating behaviors and the used spraying indicators. The determination of a new correlation factor is necessary since the iodide concentration leading to a color change (Table 3) is different for the novel indicator system. The original correlation factor \(\frac{{D_{\text{RCM}} }}{{D_{\text{RIM}} }}\) after observation of the color change boundaries by spray tests was experimentally determined by Lay as 0.987 [12]. Figure 7 shows the boundaries of the AgNO3/fluorescein chloride indicator (17 mm) as well as the original KIO3 system (17.5 mm) and the novel H2O2/K2S2O8 mixture (17.7 mm). Hence, the new correlation factor is calculated to 0.96 by dividing the respective boundaries. In any case, the slight change on the correlation factor is negligible, especially regarding rapid migration test’s coefficient of variation.
Comparison of chloride and iodide profiles and average of penetration depth Xd revealed by chloride, original iodide and novel iodide indicator after rapid migration test [7]
4 Conclusions
This paper describes the development of a novel indicator system for the determination of the penetration front of RIM tests in chloride and sulfide contaminated concretes such as GGBS containing CEM III concretes. With the help of the RIM test, it is possible to measure the rapid chloride migration coefficient of chloride-contaminated concrete using iodide as penetrating ion. The original indicator system was only applicable for CEM I type concretes and is based on the iodine starch reaction in combination with KIO3 as oxidation agent. However, this indicator is not applicable to sulfide containing concretes because the used oxidation agent KIO3 produces iodine with any reduction agent such as sulfide and generates a false positive discoloration in GGBS containing concretes.
As alternative oxidation agents H2O2 and K2S2O8 are successfully examined. Since both proposed oxidation agents show minor disadvantages (for H2O2 the iodine starch complex disappears in alkaline media while K2S2O8 takes several minutes to develop the full color) a mixture of both combines their advantages as the blue color develops immediately and stays during an appropriate time in which the user could mark the migration front with a permanent pen. Nevertheless, the use of only one of the oxidizing agents is also possible. The optimum composition of the new indicator may be a mixture of both, applying following composition for 1 L: 600 mL 3 wt% aqueous starch solution, 200 mL 50 wt% acetic acid, 100 mL 30 wt% aqueous H2O2 and 100 mL 0.21 M K2S2O8 solution.
The new indicator system is also validated for a sulfide free concrete with high basicity with the result that the new indicator system can replace the iodate system universally. For practical use H2O2 is preferable in comparison to K2S2O8 since the latter is more expensive and not as stable as H2O2 in water. In both cases, the sensitivity is higher compared to the original indicator with KIO3. Consequently, a new correlation factor \(\frac{{D_{\text{RCM}} }}{{D_{\text{RIM}} }}\) had to be calculated with a value of 0.96, which is only a small divergence to the value for the KIO3 based indicator system of 0.987 [12].
References
Torrent RJ (2018) Bridge durability design after EN standards: present and future. Struct Infrastruct Eng 15:1–13
FB (2006) Model code for service life design. International federation for structural concrete (fib)
EN 12390-11 DE (2015) Determination of the chloride resistance of concrete, unidirectional diffusion
Samson E, Marchand J, Snyder KA (2003) Calculation of ionic diffusion coefficients on the basis of migration test results. Mater Struct 36(3):156–165
Tang L (1996) Electrically accelerated methods for determining chloride diffusivity in concrete—current development. Mag Concr Res 48(176):173–179
Kessler S, Thiel C, Grosse CU, Gehlen C (2017) Effect of freeze–thaw damage on chloride ingress into concrete. Mater Struct 50(2):121
Van Noort R, Hunger M, Spiesz P (2016) Long-term chloride migration coefficient in slag cement-based concrete and resistivity as an alternative test method. Constr Build Mater 115:746–759
Spiesz P, Brouwers H (2013) The apparent and effective chloride migration coefficients obtained in migration tests. Cem Concr Res 48:116–127
NT Build (1999) Chloride migration coefficient from non-steady-state migration experiments. Nordtest Method 492:10
MCL BCoP (2012) Resistance of Concrete to Chloride Penetration (MCL)
Wasserbau BF (2012) BAWMerkblatt Chlorideindringwiderstand von Beton (MCL)
Lay S, Liebl S, Hilbig H, Schießl P (2004) New method to measure the rapid chloride migration coefficient of chloride-contaminated concrete. Cem Concr Res 34(3):421–427
Siddique R (2014) Utilization (recycling) of iron and steel industry by-product (GGBS) in concrete: strength and durability properties. J Mater Cycl Waste Manag 16(3):460–467
Ogawa S, Nozaki T, Yamada K, Hirao H, Hooton R (2012) Improvement on sulfate resistance of blended cement with high alumina slag. Cem Concr Res 42(2):244–251
Di Maio L (2016) Cement replacement materials. CSE-City Saf Energy 2:155–156
Qiu J, Charleux B, Matyjaszewski K (2001) Controlled/living radical polymerization in aqueous media: homogeneous and heterogeneous systems. Progr Polym Sci 26(10):2083–2134
EN 196-1: DE (2005) Prüfverfahren für Zement–Teil 1: Bestimmung der Festigkeit. Deutsche Fassung
Wiberg N, Dehnicke K (1996) Hollemann-Wiberg, Lehrbuch der Anorganischen Chemie. Angewandte Chemie-German Edition 108(21):2696
Acknowledgements
Open Access funding provided by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Decker, M., Grosch, R., Keßler, S. et al. Chloride migration measurement for chloride and sulfide contaminated concrete. Mater Struct 53, 90 (2020). https://doi.org/10.1617/s11527-020-01526-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1617/s11527-020-01526-4