Abstract
This paper aims to study the phase modification, reaction kinetics, mechanical properties and drying shrinkage of sodium carbonate activated slag by incorporating sodium sulfate in the activator. The results show that the reaction process is firstly controlled by CO3 2− anions, and later runs similar to that of sodium sulfate activation. Besides, the relatively unstable phase gaylussite, commonly found in the sodium carbonate activation, is not observed in the reaction products upon hybrid activation, and monosulfoaluminate rather than ettringite is identified, probably caused by the reduced aluminate-to-sulfate ratio and increased pH value. The drying shrinkage is considerably reduced by up to 41% when replacing 50 wt% sodium carbonate by sodium sulfate, most possibly attributed to the induced phase modification. Furthermore, the relationships between the phase modification and drying shrinkage, and the potentially involved chemical reaction are discussed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Environmental benefits and excellent performance of alkali activated slag cement (AAS) have promoted their fast development in recent years [1,2,3]. It is known that the reaction products, mechanical properties and durability of AAS significantly differ with different activators applied [4, 5]. The material properties of AAS together with the effect of additives [6,7,8,9] using various sole activators have been extensively reported. However, the performance of hybrid activators on activation has been rarely investigated [10,11,12], especially the durability related properties, e.g. drying shrinkage.
It is evident that both slag characteristics and activators applied essentially affect the reaction products of alkali activated slag [2, 13]. When a slag with a high MgO content is used, the secondary reaction products can be: hydrotalcite if activated by waterglass [14]; calcite, gaylussite and hydrotalcite when activated by sodium carbonate [15]; ettringite and monosulfoaluminate or no crystalline structures (depending on the Al2O3 content) if activated by sodium sulfate [16]. On the other hand, it is also known that the mechanical properties of AAS are highly depending on the applied activators when using the same solid precursors, exhibiting generally an order of Na2SiO3 > Na2CO3 > Na2SO4 > NaOH at the same curing regimes and ages [17]. Besides, the structure of the main reaction product, C–(A)–S–H gel, also alters when different activators are used. Jimenez et al. [18] reported that waterglass activation showed a high cross-linked structure with both Q 2 and Q 3 silicon sites, sodium hydroxide activation only showed Q 2 silicon sites and sodium carbonate activation showed Q 3 silicon but with the lowest Q 2 silicon values.
The application of a near-neutral salt, Na2CO3, as an activator in AAS has been attracting great interest, attributed to its advantage in terms of low alkalinity, low cost and good performance [19, 20]. Slow reaction of sodium carbonate activation has been often reported, which nevertheless can be compensated by controlling the slag characteristics [21]. However, the latest results show that the sodium carbonate activated slag suffers from high drying shrinkage [22, 23]. Jin et al. [22] found that the drying shrinkage of Na2CO3 activation (4 Na2O wt%) is about 1.15% after 90 days of curing. But they observed that increasing the sodium carbonate content (6% Na2O wt%) leads to a significant reduction on the shrinkage development, while the incorporation of MgO (<5 wt%) only slightly declines the drying shrinkage. It should be noted that gaylussite, commonly found in sodium carbonate activation as a secondary reaction product, is not a stable phase and will gradually decompose and convert to other phases [15].
The decomposition process may potentially not be positive for the stability of the host matrix [24].
The application of hybrid activators has also been reported to modify the reaction products. Bernal et al. [12] evaluated the slag activated by a blended sodium carbonate and sodium silicate activator, and found pirssonite (Na2Ca(CO3)2·2H2O) and hydroxysodalite (Na8Al6Si6O24(OH)2(H2O)2) instead of gaylussite as the main secondary products. The present authors also found the absence of gaylussite when the activator of sodium carbonate was mixed with a certain amount of waterglass [11]. As the shrinkage is mainly controlled by the amorphous and unstable phases, the effect of crystalline structures is not prominent. In this case, it is reasonable to assume that by modifying the reaction products of the sodium carbonate activation, the shrinkage problem can be, to some extent, tackled.
A preferable option would be introducing expanding sources into the matrix, which will benefit the shrinkage [25]. Ettringite is known as an expanding source in ordinary Portland cement systems, and it has also been reported in the alkali sulfate activated slag [26, 27]. Mobasher et al. [27] evaluated the structure of an alkali sulfate activated slag cement, and found that ettringite, C–A–S–H, and a hydrotalcite-like Mg–Al layered double hydroxide (LDH) are the main reaction products. However, Bernal [16] also mentioned that the secondary reaction products are highly depending on the content of MgO and Al2O3. A high content of MgO and/or low content of Al2O3 favors the formation of poorly crystalline LDHs and AFm type phases, while a reversed condition leads to the generation of monosulfoaluminate and ettringite. It should be noted that both hydrotalcite and ettringite basically belong to the group of layered double hydroxides, while their synthesis conditions are different [16, 28]. In this case, by combining the alkaline solutions of sodium carbonate and sodium sulfate as the activator, it is possible to enhance the crystallinity by forming new LDHs and avoiding the formation of gaylussite, which consequently will reduce the shrinkage.
The present research aims to study the effect of sodium sulfate incorporation in the sodium carbonate activated slag system on the reaction kinetics, mechanical properties and drying shrinkage. The microstructure modification caused by the incorporated sodium sulfate is analyzed applying X-ray diffraction (XRD), Scanning electron microscope (SEM) and Fourier transform infrared spectroscopy (FTIR). Furthermore, the relationships between phase modification and drying shrinkage, and the potentially involved chemical reaction are discussed.
2 Materials and experiments
2.1 Materials
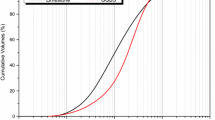
Ground granulated blast furnace slag (GGBS) (supplied by ENCI, the Netherlands) is applied in this study. The chemical composition of the GGBS determined by X-ray Fluorescence is shown in Table 1 and the particle size distribution measured by a laser granulometer (Mastersizer 2000) is depicted in Fig. 1. Sodium carbonate and sodium sulfate, both in powder form with analytical grade, are used as alkali sources (Table 2). Sodium carbonate activation is designated as “SC”, sodium sulfate activation “SS”, hybrid activators activation without limestone powder (“SCS”) and with limestone powder (“SCSLP”) in the following sections for clear interpretations of the results. The activators were first prepared and cooled down to room temperature (20 ± 1 °C) prior to further application.
2.2 Experiments
Paste samples were prepared according to EN 196-1. Prisms (40 × 40 × 160 mm3) were cast for strength measurement and a plastic foil was used to cover the surface of the cast specimens to prevent the moisture loss. The samples were stored in a climate chamber at room temperature (20 ± 2 °C) with the relative humidity of >99% till the ages of testing. The following experiments were performed:
-
The compressive strength of the samples were tested at the curing ages of 3, 7 and 28 days, respectively, according to EN 196-1. The presented results were the average of 6 samples.
-
An isothermal conduction calorimeter (TAM AIR Calorimeter, set at 20 °C) was used to measure the heat evolution of the samples during the activation. It should be noted that the recorded data during the initial ~45 min might not be reliable because of the possible unstable environment caused by the sample preparation process.
-
The X-ray diffraction (XRD) analysis was conducted using a Siemens/Bruker D5000 X-ray Powder Diffraction system with a Cu tube (20 kV, 10 mA) and the setting of: scanning range 5°–55°, step of 0.02° and measuring time 30 s/step.
-
A PerkinElmer Frontier™ IR/FIR Spectrometer using the attenuated total reflection (ATR) method (GladiATR) at a resolution of 4 cm−1 was used for the FTIR measurement.
-
The microscopic analysis was performed using a JSM-IT100 InTouchScope™ Scanning Electron Microscope (SEM).
-
The drying shrinkage was determined to samples stored in a climate chamber (20 ± 1 °C and RH 65%) based on DIN 52450: 1985. A digital length comparator (±0.001 mm) was adopted to measure the linear dimension variation of the samples along the longitudinal axis.
3 Results and discussion
3.1 Mechanical properties
Figure 2 presents the strength development of the samples (see Table 2) at the curing ages of 3, 7 and 28 days, respectively. As can be seen, the compressive strength of all samples increase with the increase of curing age. Sodium carbonate activation is often reported to be slow. Nevertheless, slag characteristics [21] have been found to be an important factor in controlling the reaction kinetics, and a high early strength is achievable by adjusting the fineness of slag particles. In this study, a compressive strength of 28.89 MPa after 3 days of curing is observed for the sodium carbonate activated slag. The sodium sulfate activated slag shows a relatively lower strength at all ages, compared to that of sodium carbonate activation. The result is in line with previous findings that the activation effect of Na2CO3 is better than Na2SO4 with respect to mechanical properties [17]. However, the strength development of mixture SS is faster than most of the previously published results [17, 27]. Mobasher et al. [27] studied the activation effect of Na2SO4 and reported a compressive strength of 17 MPa after 28 days of curing. Wang et al. [17] found that the strength of sodium sulfate activation is highly depending on the slag type, and they obtained 3, 20 and 30 MPa with three slags, acid, neutral and basic slag, respectively, after 28 days of curing. Besides, Rashad et al. [26] reported that the slag fineness played a significant role on the strength development, while the dosage only influenced the early age reaction. In this case, the relatively high compressive strength of Na2SO4 activated slag can be attributed to the fine slag particles and its high basicity coefficient (K b = (CaO + MgO)/(SiO2 + Al2O3) = 1.50, i.e. basic slag). It should be noted that the samples of the mixture SS broken into pieces after 28 days of curing (RH > 99%), while the samples cured at RH 65% only showed some slightly visible cracks. One possible reason could be that a high humidity curing condition modifies the formation of reaction products which increases the internal stress, i.e. a high humidity condition accelerates the formation of ettringite. The sample activated by the hybrid activator could potentially have the same issue after long-term curing, nevertheless it was not observed in the current study after 6 months of curing [29]. Nevertheless, this phenomenon requires further investigation.
When the slag was activated by the hybrid activators of Na2CO3 (2 Na2O wt%) and Na2SO4 (2 Na2O wt%) (Mixture SCS), the compressive strength of the specimens are more close to the strength development of Na2SO4 activation. The low early strength (3 days) could be attributed to the unfinished heat release, as shown in the calorimetric results. As the saturation limitation of CaCO3 is lower than that of C–A–S–H gel and CaSO4 [30], the reaction will be firstly controlled by CO3 2− anions and then the pore solution condition is similar to that sodium sulfate activation. In this case, the precipitation environment of calcium silicate hydrates in the hybrid activator’s activation system is more close to that of sodium sulfate activation. The C–A–S–H gel is the main reaction product of alkali activated slag, which could possibly be one of the reasons to explain the strength development of hybrid activator’s activation. Furthermore, the influence of additive limestone powder (LP) on the strength was also studied and the results show that the incorporation of LP slightly weakens the mechanical properties, i.e. with 50 vol% of slag replaced by LP the compressive strength at 28 days only declines 16.0%. The similar results were found in previous findings in sodium carbonate activated slag system [7, 24]. Rakhimova et al. [7] reported that the strength development of samples were not weakened up to 50% replacement of slag by LP. It should be noted that, due to the dilution effect, the delayed reaction of samples containing LP is also contributing to the slow strength development.
3.2 Reaction kinetics analysis
Figure 3 depicts the heat release of all mixtures with different activators and the process generally can be classified into five stages which is in accordance with previous researches [14, 31,32,33]. The time to reach reaction peak (TRRP) of sodium carbonate activation is shortened to around 50 h compared to previous reports [15, 34], which is in line with our previous study that the reaction can be significantly accelerated by controlling the slag characteristics [21]. As for the activation of sodium sulfate, the reaction is slightly different from previous studies [26, 27] by giving an intensive reaction peak after 8 h of casting. Mobasher et al. [27] reported very long induction periods (between 100 and 125 h, measured at 25 °C) with the dosages of 0.8, 1.7 and 5.1 (Na2O equivalent). While Rashad et al. [26] found that the reaction is highly depending on the slag fineness (2500 and 5000 cm2/g) and Na2SO4 dosage (1 and 3% (Na2O equivalent)) and in general a dormant period of 25–70 h (tested at 40 °C) is shown. In this case, the accelerated reaction of sodium sulfate activation in the present study can be explained by the different slag characteristics and relatively high dosage. It is noticed that the solubility of CaCO3 (0.0013 g/100 ml at 25 °C) is much lower than CaSO4 (0.21 g/100 ml at 25 °C) in water, while the solubility of CaSO4 is also higher than C–A–S–H gel at the pH of 12–13 [26]. In this case, the reaction of sodium sulfate activated slag should be faster than sodium carbonate activation when the same amount of activators is used, which is in line with the current result (Fig. 3) and previous reports when similar slags were applied [15, 27].
On the other hand, the reaction of slag activated by hybrid activators, Na2CO3 and Na2SO4 (2 + 2 Na2O wt%), is similar to that of sodium carbonate activation but shows a slightly slower reaction rate and lower intensity. As discussed by Bernal et al. [15], the sodium carbonate activation is a solubility controlled reaction, i.e. CO3 2− concentration in the pore solution. Because the saturation limit of CaCO3 is lower than C–(A)–S–H gel, the precipitation of CaCO3 will happen first. In this case, the early reaction is primarily controlled by CO3 2− and then similar to that of SO4 2− activation. This explains that the reaction process of slag activated by the hybrid activators is similar to that of sodium carbonate, while the strength development is similar to sodium sulfate activation because the formation of C–(A)–S–H gel is similar to the condition of sodium sulfate activation. Moreover, the reaction of samples activated by the hybrid activator is ~8 h later than sodium carbonate activation, which indicates the potential inhibiting effect when both CO3 2− and SO4 2− anions are present. However, the hybrid activator’s activation system is so complex that further investigation is still required to obtain deeper insight. Furthermore, similar to a previous research [23], the reaction is significantly delayed after replacing 50 vol% of GGBS by limestone powder. Due to the dilution effect, sufficient Ca2+ requires more dissolution time to be precipitated with CO3 2− and SO4 2−, which, in turn, leads to a slower and less intensive reaction.
3.3 Reaction products characterization
Figure 4 presents the characterized reaction products of the samples at the curing age of 28 days. As can be seen, the main reaction products are highly depending on the activators applied. When the slag is activated solely by sodium carbonate, the main secondary reaction products are calcite, gaylussite and hydrotalcite (when the MgO content in the slag is high [14]), which is in line with previous findings [15, 35]. Besides, hemicarboaluminate (PDF #00-036-0129), also named hemicarbonate, is also observed [35]. The existence of this phase indicates that the system is abundant with aluminum because the Mg content is not sufficient to consume all Al2O3, forming hydrotalcite [35,36,37]. Similar phenomenon was also reported by Ke et al. [35], which is due to the preference of forming layered double hydroxide structures. When switching the activator to sodium sulfate, the secondary reaction products change to ettringite. However, it should be noted that monosulfoaluminate could also be possibly existing as identified by Mobasher et al. [27] applying XRD together with solid-state 27Al MAS NMR spectroscopy. They found the possible existence of hydrotalcite-like phase in the reaction products, which were not observed in the XRD pattern. Bernal [16] reported that the reaction products of sodium sulfate activation are considerably depending on the MgO and Al2O3 content in the raw slags. A high MgO and low Al2O3 content lead to the formation of poorly crystalline hydrotalcite-like phase and/or AFm type phases rather than ettringite and/or monosulfoaluminate.
On the other hand, when the slag is activated by the hybrid activators of Na2CO3 and Na2SO4, hydrotalcite and monosulfoaluminate are identified as the main secondary reaction products, while ettringite is not presented. Besides, gaylussite, commonly found as a secondary reaction product in sodium carbonate activation, is not observed either. In this case, it is reasonable to conclude that the hybrid activators change the chemical reaction process, leading to the formation of different phases. The potentially involved chemical reactions will be discussed in Sect. 3.5. Furthermore, 50 vol% of slag is replaced by limestone powder and the presence of limestone powder leads to the formation of a new phase, natron (Na2CO3·10H2O). It is worth to note that natron is chemically unlikely to be the initial reaction product or the residual activators. As discussed above (Fig. 3), the early age reaction process is mainly controlled by the CO3 2− anions, while the later stage reaction is similar to SO4 2− activation. Sufficient CO3 2− anions should be consumed prior to further reaction, e.g. precipitation of C–(A)–S–H. In this case, natron should be converted from the decomposition of gaylussite, as discussed in the Introduction. The same phenomenon was also found in our previous study [24] that a high dosage of limestone powder in the sodium carbonate activated slag system leads to the formation of natron.
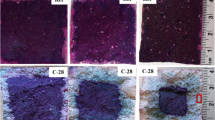
Figure 5 shows the SEM pictures of all samples at the curing age of 28 days. As can be seen, the morphology of mixture SC (Table 2) shows a layered crystalline structure embedded in the matrix, which is likely to be a LDH [38] as revealed by the XRD result (Fig. 4), i.e. hydrotalcite and/or hemicarboaluminate. While in the case of sodium sulfate activation (mixture SS), the morphology shows a crystalline structure of needle shape, i.e. ettringite. It should be noted that gypsum also shows a needle shape that could be coexisting in the system. Mobasher et al. [39] identified the traces of unreacted Na2SO4 after 6 months of curing via 23Na MAS NMR spectroscopy. In the case of the hybrid activator activation (mixture SCS), hydrotalcite was also observed, which is in line with the analysis that the ion exchanged ability of CO3 2− is higher than SO4 2−, so preferred to be incorporated in the layered double hydroxide structures (LDHs). Layered double hydroxide structure was also found in the mixture SCSLP after incorporating 50 vol% limestone powder.
Figure 6 presents the FTIR spectra of samples activated by different activators at the curing age of 28 days. As can be seen, the vibration of free water in the reaction products was observed in all samples, indicated by the broad peaks at approximate 3315 and 1644 cm−1. Depending on the activators applied, CO3 2− and SO4 2− anions are also identified in the samples as shown in the spectra. The peaks centred at about 1410 and 1493 cm−1 are assigned to be the vibrations of v 3[CO3 2−], which can be calcium carbonate or hydrotalcite [40,41,42]. A small peak at this position was also observed in the mixture activated by pure sodium sulfate, possibly due to a slight carbonation or originated from slags. Besides, all spectra give intensive peaks at around 950 cm−1, indicating the formation of C–(A)–S–H gel. However, a slightly lower position was observed for the mixture SC (944 cm−1). Puertas et al. [43], Palacios and Puertas [44] and Puertas and Carrasco [40] found that the position in the range between 900 and 1000 cm−1 is highly corresponding to the chemical composition of calcium silicate hydrate, i.e. Ca/Si ratio. A different wavenumber indicates a different chemical proportion (Si/Ca ratio) [40, 43, 44]. In this case, the low position indicates that the formation of C–(A)–S–H gel is mainly controlled by SO4 2− anions as the gel structure is more similar to that of samples activated by sodium sulfate. This phenomenon is in consistence with the analysis above. Nevertheless, the utilization of using FTIR to check the microstructure, i.e. chemical composition of calcium silicate hydrate still requires deeper investigation and is open for discussion.
3.4 Drying shrinkage analysis
Figure 7 presents the drying shrinkage of the mixtures activated with different activators. Similar to previous finding [22], sodium carbonate activation presents a drying shrinkage of 0.75% after about 43 days of curing. However, the shrinkage of sodium sulfate activated slag is much lower, giving 0.37%, which is around 50% lower than that of sodium carbonate activation. Up till now, only a few available investigations [16, 26, 27, 45] reported the sodium sulfate activation, and the shrinkage was rarely investigated. However, the main secondary reaction products are ettringite and monosulfoaluminate that are stable phases, also known as expanding sources in ordinary cement systems. While in the case of sodium carbonate activation, gaylussite is not stable and will gradually decompose, releasing free water. As a result, the drying shrinkage of the sodium sulfate activation is lower compared to that of sodium carbonate activated slag.
When the slag is activated by the hybrid activators, the drying shrinkage (0.44%) is slightly higher than that of sodium sulfate activation probably attributed to the absence of the expanding source ettringite, but much lower than that of sodium carbonate activation. As can be seen in Fig. 4, the main secondary reaction products are calcite, monosulfoaluminate and hydrotalcite, which are all relatively stable phases under ambient conditions, while the unstable phase gaylussite (Eq. 1), commonly found in only sodium carbonate activation and will completely convert to other phases at the ambient condition [15], is not presented. In this case, the improved drying shrinkage could be attributed to the phase modification. Furthermore, after replacing 50 vol% slag by limestone powder in the hybrid activation system, the drying shrinkage is considerably increased to 1.21%, which is about 2.8 times higher than that of samples without limestone powder. In our previous study [23], the present authors found that the amount of mesopores and macropores increases with the increase of limestone powder, while that of gel pores decreases due to the reduced amount of C–(A)–S–H gel (LP > 30 vol%). Due to the dilution effect, more free water is available in the pores and will be dried out (>~5 nm) according to the Kelvin–Laplace equation [46], which consequently enlarges the drying shrinkage. It should be noted that in waterglass activated slag-fly ash blenders system, Gao et al. [47] found that the drying shrinkage of samples decreased with the decrease of slag content. In this case, the decomposition process of gaylussite and natron releasing free water to pores can also contribute to the enlarged drying shrinkage. Nevertheless, further investigation is still required in order to acquire more quantitative information.
3.5 Phase modification analysis
Up till now, attempts on the hybrid activators were rarely explored [10,11,12]. From the chemical point of view, as shown by the XRD results, the main reaction products of slag activated by hybrid activators are slightly different from the activation by a sole activator. The existence of hemicarboaluminate in the sodium carbonate confirms the excess of Al3+ ions in the system. Besides, hydrotalcite instead of ettringite is observed in the hybrid activation system (Fig. 4). Hydrotalcite-like structure is a typical layered double hydroxides (LDHs) and widely synthesized in the laboratory using a wide variety of methods [28]. Considering the coexistence of SO4 2− and CO3 2− anions, the formation of LDHs at the current condition is very similar as the one prepared using the ion-exchange method. He et al. [28] reviewed the preparation of LDHs and reported that the exchange ability of anions increases with the increasing charge and decreasing ionic radius, i.e. CO3 2− > SO4 2−. Besides, Bontchev et al. [48] also found that the order of ion exchange preference was not affected by the co-intercalation of a second anion. As a result, in the hybrid activators’ system, CO3 2− anions are preferred to be firstly incorporated in the structure LDHs generating hydrotalcite, while the remaining Al3+ will react with SO4 2−, forming monosulfoaluminate instead of hemicarboaluminate.
It should be noted that monosulfoaluminate (Ca4Al2(SO4)(OH)12·6H2O) instead of ettringite (Ca6Al2(SO4)3(OH)12·26H2O) was observed in the samples activated by the hybrid activators. One possible reason could be related to the reduced amount SO4 2− anions provided by the activators. Clark and Brown [49] found that aluminate-to-sulfate ratio dominates the reaction and ettringite was the only crystalline phase ultimately formed at a molar ratio of 1:3, regardless of the pH and temperature. However, at a high aluminate-to-sulfate ratio (1:1), sodium-substituted monosulfoaluminate, also named U-phase, became the dominant phase after increasing the NaOH concentration [50]. Gabrisovd et al. [51] also found that increasing the pH value from 11.6 to 12.5 changed the reaction products from ettringite to monosulfoaluminate. Rashad et al. [26] monitored the pH value of the pastes made with slag activated by 1 and 3% Na2O equivalent of Na2SO4 and found that the pH value is fluctuated at around 12.5, which is considerably lower than that of other activators. In our previous study, the pH of sodium carbonate activated slag was checked by immersing pH sensor into the fresh paste which quickly increased up to around 13.5 after 2 h of mixing. In this case, the preference of forming monosulfoaluminate could also probably due to the increased pH value. Nevertheless, the hybrid activator’s activation is so complex that further investigation is still needed to reveal/optimize its benefits with respect to the durability related modifications.
4 Conclusions
The effect of sodium sulfate on the phase modification, mechanical properties and drying shrinkage of sodium carbonate activated slag is studied in the present paper. The microstructure changes and chemical reaction process are discussed. Based on the obtained results, the following conclusions can be drawn:
-
1.
The reaction of slag activated by hybrid activators is firstly controlled by CO3 2− anions, and then is similar to that of sodium sulfate activation.
-
2.
The main secondary reaction products of hybrid activators activation are calcite, hydrotalcite and monosulfoaluminate.
-
3.
Hydrotalcite instead of hydrotalcite-SO4 2− is preferred to be formed in the hybrid activation system because of the higher ion exchange ability of CO3 2− compared to SO4 2−.
-
4.
Monosulfoaluminate is preferred to be precipitated rather than ettringite in the hybrid activators activation system, attributed to the increased aluminate-to-sulfate ratio and pH value compared to pure sodium sulfate activation.
-
5.
The drying shrinkage of slag activated by hybrid activator is about 41% lower than that of plain carbonate activation mostly attributed to the phase modification, i.e. the main secondary reaction products are changed from calcite, gaylussite and hydrotacite to calcite, monosulfoaluminate and hydrotalcite. However, further investigation is still needed to acquire more quantitative information.
References
Palomo A, Shi C, Jimenez AF (2011) New cements for the 21st century: the pursuit of an alternative to Portland cement. Cem Concr Res 41:750–763. doi:10.1016/j.cemconres.2011.03.016
Provis JL, van Deventer JSJ (n.d) Alkali activated materials: state-of-the-art report. RILEM TC 224-AAM
Huiskes DMA, Keulen A, Yu QL, Brouwers HJH (2015) Design and performance evaluation of ultra-lightweight geopolymer concrete. Mater Des 89:516–526. doi:10.1016/j.matdes.2015.09.167
Shi C, Day RL (1996) Some factors affecting early hydration of alkali-slag cements. Cem Concr Res 26:439–447. doi:10.1016/S0008-8846(96)85031-9
Jimenez AF, Puertas F (2001) Setting of alkali-activated slag cement. Influence of activator nature. Adv Cem Res 13:115–121. doi:10.1680/adcr.2001.13.3.115
Gao X, Yu QL, Brouwers HJH (2015) Reaction kinetics, gel character and strength of ambient temperature cured alkali activated slag–fly ash blends. Constr Build Mater 80:105–115. doi:10.1016/j.conbuildmat.2015.01.065
Rakhimova NR, Rakhimov RZ, Naumkina NI, Khuzin AF, Osin YN (2016) Influence of limestone content, fineness, and composition on the properties and microstructure of alkali-activated slag cement. Cem Concr Compos 72:268–274. doi:10.1016/j.cemconcomp.2016.06.015
Gao X, Yu QL, Brouwers HJH (2015) Properties of alkali activated slag–fly ash blends with limestone addition. Cem Concr Compos 59:119–128. doi:10.1016/j.cemconcomp.2015.01.007
Gao X, Yu QL, Brouwers HJH (2015) Characterization of alkali activated slag–fly ash blends containing nano-silica. Constr Build Mater 98:397–406. doi:10.1016/j.conbuildmat.2015.08.086
Collins F, Sanjayan JG (1998) Early age strength and workability of slag pastes activated by NaOH and Na2CO3. Cem Concr Res 28:655–664
Yuan B, Yu QL, Brouwers HJH (2015) Reaction kinetics, reaction products and compressive strength of ternary activators activated slag designed by Taguchi method. Mater Des 86:878–886. doi:10.1016/j.matdes.2015.07.077
Bernal SA, San Nicolas R, Provis J, van Deventer JS (2015) Alkali-activated slag cements produced with a blended sodium carbonate/sodium silicate activator. Adv Cem Res. doi:10.1680/adcr.15.00013
Garcia-Lodeiro I, Fernández-Jiménez A (2015) 02—An overview of the chemistry of alkali-activated cement-based binders. In: Handbook of alkali-activated cements mortars and concretes. doi:10.1533/9781782422884.1.19
Bernal SA, Nicolas RS, Myers RJ, Mejia De Gutierrez R, Puertas F, Van Deventer JSJ, Provis JL (2014) MgO content of slag controls phase evolution and structural changes induced by accelerated carbonation in alkali-activated binders. Cem Concr Res 57:33–43. doi:10.1016/j.cemconres.2013.12.003
Bernal SA, Provis JL, Myers RJ, San Nicolas R, van Deventer JS (2014) Role of carbonates in the chemical evolution of sodium carbonate-activated slag binders. Mater Struct 48:517–529. doi:10.1617/s11527-014-0412-6
Bernal SA (2016) Advances in near-neutral salts activation of blast furnace slags. RILEM Tech Lett 1:39–44
Wang S, Scrivener KL, Pratt PL (1994) Factors affecting the strength of alkali-activated slag. Cem Concr Res 24:1033–1043
Jimenez A, Puertas F, Sobrados I, Sanz J (2003) Structure of calcium silicate hydrates formed in alkaline-activated slag: influence of the type of alkaline activator. J Am Ceram Soc 86:1389–1394. doi:10.1111/j.1151-2916.2003.tb03481.x
Moseson AJ, Moseson DE, Barsoum MW (2012) High volume limestone alkali-activated cement developed by design of experiment. Cem Concr Compos 34:328–336. doi:10.1016/j.cemconcomp.2011.11.004
Yuan B, Straub C, Segers S, Yu QL, Brouwers HJ (2017) Sodium carbonate activated slag as cement replacement in autoclaved aerated concrete. Ceram Int 43:6039–6047. doi:10.1016/j.ceramint.2017.01.144
Yuan B, Yu QL, Brouwers HJH (2017) Evaluation of slag characteristics on the reaction kinetics and mechanical properties of Na2CO3 activated slag. Constr Build Mater 131:334–346. doi:10.1016/j.conbuildmat.2016.11.074
Jin F, Gu K, Al-Tabbaa A (2014) Strength and drying shrinkage of slag paste activated by sodium carbonate and reactive MgO. Constr Build Mater 81:58–65. doi:10.1016/j.conbuildmat.2013.10.081
Yuan B, Yu QL, Dainese E, Brouwers HJH (2017) Autogenous and drying shrinkage of sodium carbonate activated slag altered by limestone powder incorporation. Constr Build Mater 153:459–468. doi:10.1016/j.conbuildmat.2017.07.112
Yuan B, Yu QL, Brouwers HJH (2017) Assessing the chemical involvement of limestone powder in sodium carbonate activated slag. Mater Struct 50:136
Yuan X, Chen W, Lu Z, Chen H (2014) Shrinkage compensation of alkali-activated slag concrete and microstructural analysis. Constr Build Mater 66:422–428. doi:10.1016/j.conbuildmat.2014.05.085
Rashad AM, Bai Y, Basheer PAM, Milestone NB, Collier NC (2013) Hydration and properties of sodium sulfate activated slag. Cem Concr Compos 37:20–29. doi:10.1016/j.cemconcomp.2012.12.010
Mobasher N, Bernal SA, Provis JL (2016) Structural evolution of an alkali sulfate activated slag cement. J Nucl Mater 468:97–104. doi:10.1016/j.jnucmat.2015.11.016
He J, Wei M, Li B, Kang Y, Evans DG, Duan X (2006) Preparation of layered double hydroxides. Layer Double Hydroxides 119:89–119. doi:10.1007/430_006
Xu H, Provis JL, Van Deventer JSJ, Krivenko PV (2008) Characterization of aged slag concretes. ACI Mater J 105:131–139
Sheikholeslami R, Ng M (2001) Calcium sulfate precipitation in the presence of nondominant calcium carbonate: thermodynamics and kinetics. Ind Eng Chem Res 40:3570–3578. doi:10.1021/ie000781c
Ravikumar D, Neithalath N (2012) Reaction kinetics in sodium silicate powder and liquid activated slag binders evaluated using isothermal calorimetry. Thermochim Acta 546:32–43. doi:10.1016/j.tca.2012.07.010
Ben Haha M, Lothenbach B, Le Saout G, Winnefeld F (2012) Influence of slag chemistry on the hydration of alkali-activated blast-furnace slag—Part II: Effect of Al2O3. Cem Concr Res 42:74–83. doi:10.1016/j.cemconres.2011.08.005
Yu QL, Brouwers HJH (2012) Development of a self-compacting gypsum-based lightweight composite. Cem Concr Compos 34:1033–1043. doi:10.1016/j.cemconcomp.2012.05.004
Yuan B, Yu QL, Brouwers HJH (2015) Time-dependent characterization of Na2CO3 activated slag. Cem Concr Compos (Under review)
Ke X, Bernal SA, Provis JL (2016) Controlling the reaction kinetics of sodium carbonate-activated slag cements using calcined layered double hydroxides. Cem Concr Res 81:24–37. doi:10.1016/j.cemconres.2015.11.012
Whittaker M, Zajac M, Ben Haha M, Bullerjahn F, Black L (2014) The role of the alumina content of slag, plus the presence of additional sulfate on the hydration and microstructure of Portland cement-slag blends. Cem Concr Res 66:91–101. doi:10.1016/j.cemconres.2014.07.018
Matschei T, Lothenbach B, Glasser FP (2007) The AFm phase in Portland cement. Cem Concr Res 37:118–130. doi:10.1016/j.cemconres.2006.10.010
Pollmann Herbert (2010) Mineralisation of wastes and industrial residues. Shaker Verlag GmbH, Germany
Mobasher N, Bernal SA, Hussain OH, Apperley DC, Kinoshita H, Provis JL (2014) Characterisation of Ba(OH)2–Na2SO4-blast furnace slag cement-like composites for the immobilisation of sulfate bearing nuclear wastes. Cem Concr Res 66:64–74. doi:10.1016/j.cemconres.2014.07.006
Puertas F, Carrasco MT (2014) Use of glass waste as an activator in the preparation of alkali-activated slag. Mechanical strength and paste characterisation. Cem Concr Res 57:95–104. doi:10.1016/j.cemconres.2013.12.005
Abdalqader AF, Jin F, Al-Tabbaa A (2015) Characterisation of reactive magnesia and sodium carbonate-activated fly ash/slag paste blends. Constr Build Mater 93:506–513. doi:10.1016/j.conbuildmat.2015.06.015
Garcia Lodeiro I, Macphee DE, Palomo A, Fernandez AJ (2009) Effect of alkalis on fresh C–S–H gels. FTIR analysis. Cem Concr Res 39:147–153. doi:10.1016/j.cemconres.2009.01.003
Puertas F, Jimenez AF, Blanco-Varela MT (2004) Pore solution in alkali-activated slag cement pastes. Relation to the composition and structure of calcium silicate hydrate. Cem Concr Res 34:139–148. doi:10.1016/S0008-8846(03)00254-0
Palacios M, Puertas F (2006) Effect of carbonation on alkali-activated slag paste. J Am Ceram Soc 89:3211–3221. doi:10.1111/j.1551-2916.2006.01214.x
Rashad AM, Bai Y, Basheer PAM, Collier NC, Milestone NB (2012) Chemical and mechanical stability of sodium sulfate activated slag after exposure to elevated temperature. Cem Concr Res 42:333–343. doi:10.1016/j.cemconres.2011.10.007
Ye H, Radlinska A (2016) Shrinkage mechanisms of alkali-activated slag. Cem Concr Res 88:126–135. doi:10.1016/j.cemconres.2016.07.001
Gao X, Yu QL, Brouwers HJH (2016) Assessing the porosity and shrinkage of alkali activated slag-fly ash composites designed applying a packing model. Constr Build Mater 119:175–184. doi:10.1016/j.conbuildmat.2016.05.026
Bontchev RP, Liu S, Krumhansl JL, Voigt J, Nenoff TM (2003) Synthesis, characterization, and ion exchange properties of hydrotalcite Mg6Al2(OH)16(A) x (A′)−x , 4H2O (A, A′ = Cl−, Br−, I−, and NO3−, 2 > x > 0) derivatives. Chem Mater 15:3669–3675
Clark B, Brown P (1999) The formation of calcium sulfoaluminate hydrate compounds. Part I. Cem Concr Res 29:1943–1948. doi:10.1016/S0008-8846(99)00200-8
Clark BA, Brown PW (2000) Formation of calcium sulfoaluminate hydrate compounds. Part II. Cem Concr Res 30:1943–1948. doi:10.1016/S0008-8846(99)00234-3
Gabrisova A, Havlica J, Sahu S (1991) Stability of calcium sulphoaluminate hydrates in water solutions with various pH values. Cem Concr Res 21:1023–1027. doi:10.1016/0008-8846(91)90062-M
Acknowledgements
This research was carried out under the support of China Scholarship Council and the Department of the Built Environment at Eindhoven University of technology. Mr. E. Dainese is acknowledged for the help with the mechanical property and drying shrinkage measurement, and Mr. Lihua Shen M.Sc. for performing the SEM analysis. Furthermore, the authors wish to express their gratitude to the following sponsors of the Building Materials research group at TU Eindhoven: Rijkswaterstaat Grote Projecten en Onderhoud, Graniet-Import Benelux, Kijlstra Betonmortel, Struyk Verwo, Attero, Enci, Rijkswaterstaat Zee en Delta—District Noord, Van Gansewinkel Minerals, BTE, V.d. Bosch Beton, Selor, GMB, Icopal, BN International, Eltomation, Knauf Gips, Hess AAC Systems, Kronos, Joma, CRH Europe Sustainable Concrete Centre, Cement&BetonCentrum, Heros, Inashco, Keim, Sirius International, Boskalis, NNERGY, Tata Steel, Millvision, Sappi, Studio Roex and Van Berlo Group (in chronological order of joining).
Funding
This research was carried out under the fund of China Scholarship Council and support of the Department of the Built Environment in Eindhoven University of Technology.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
B. Yuan has received scholarship from China Scholarship Council and support from the Department of the Built Environment in Eindhoven University of Technology. The authors declare that they have no conflict of interest.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Yuan, B., Yu, Q.L. & Brouwers, H.J.H. Phase modification induced drying shrinkage reduction on Na2CO3 activated slag by incorporating Na2SO4 . Mater Struct 50, 220 (2017). https://doi.org/10.1617/s11527-017-1088-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1617/s11527-017-1088-5