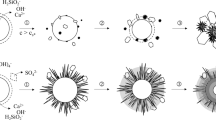
Abstract
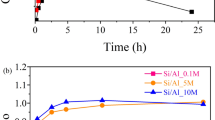
We studied the physicochemical properties of quicklimes derived from typical carbonate rocks, focusing on variations in chemical composition and their effects on reactivity. Samples were selected based upon their composition (two Mg-rich and two Ca-rich samples), freshness (scarce secondary alteration features), texture (two common Hellenic limestones and two marbles), and similarity to materials commonly used in industrial practice. We characterized the samples in detail by chemical analysis, X-ray diffraction, N2-BET, scanning electron microscopy, petrography, TG/DTA and EN 459-2 testing, and examined correlations between measured properties and reactivity. Surprisingly, specific surface area (SSA) is found not to be a determinant factor for reactivity. As expected, chemical composition has an important role in the sintering and slaking mechanisms. Impurities such as Fe2O3 and K2O facilitate, while MgO inhibits, sintering. Mg-rich quicklime, with impurities in trace amounts, calcined at 1,200 °C, has SSA of 11.9 m2/g while Ca-rich quicklime, with considerable impurities, has SSA of 0.6 m2/g. Variations in the slaking behavior, monitored by reactivity tests, were identified and related to the properties of the quicklimes. The variations can be understood in the context of a proposed five-step slaking mechanism. Slaking curves are found to be insensitive to source-rock texture; they are similar for the two sets of Mg-rich and the two sets of Ca-rich quicklimes, respectively. MgO is found to improve hydration resistance of the material, leading to more complex slaking curves. A general firing scheme is proposed based on the investigated material properties.








Similar content being viewed by others
References
Al-Shawabkeh A, Matsuda H, Hasatani M (1997) Dry, high-temperature de-SO2 and de-H2S via treated calcium-based materials. Energy Convers Manag 38(10):1389–1397
Baldo JB, Bradt RC (1988) Grain growth of lime and periclase phase in synthetic doloma. J Am Ceram Soc 71:720–725
Baziotis I, Leontakianakos G, Proyer A, Lee H, Tsimas S (2011) Physico-chemical properties of different carbonate rocks: are they highly enough to control lime reactivity? Int J Chem 3(2):187–197
Beruto DT, Searcy AW (1974) Use of the Langmuir method for the kinetic studies of decomposition reaction: calcite. J Chem Soc Faraday Trans 70:2145
Beruto DT, Searcy AW (1976) Calcium oxides of high reactivity. Nature 263:221–222
Beruto DT, Searcy AW, Botter R (1984) The thermodynamics and kinetics of carbon dioxide chemisorption on calcium. J Phys Chem 88:4052–4055
Beruto DT, Searcy AW, Botter R, Giordani M (1993) Thermodynamics and kinetics of H2O(V) chemisorption and solubility in nanometric and single crystal MgO particles during sintering. J Phys Chem 97:9201–9206
Beruto DT, Searcy AW, Mun G-K (2004) Microstructure, kinetic, thermodynamic analysis for calcite decomposition: free surface and powder bed experiments. Thermochim Acta 424:99–109
Beruto DT, Botter R, Cabella R, Lagazzo A (2010) A consecutive decomposition–sintering dilatometer method to study the effect of limestone impurities on lime microstructure and its water reactivity. J Eur Ceram Soc 30:1277–1286
Borgwardt RH (1985) Calcination kinetics and surface area of dispersed limestone particles. AIChE J 31:103–110
Botha A, Strydom CA (2001) Preparation of a magnesium hydroxy carbonate from magnesium hydroxide. Hydrometallurgy 62:175–183
Botha A, Strydom CA (2003) DTA and FT-IR analysis of the rehydration of basic magnesium carbonate. J Therm Anal Calorim 71:987–995
Boynton WV (1980) Chemistry and technology of lime and limestone, 2nd edn. Wiley, New York British Lime Association, 2012. http://www.britishlime.org.uk/. Accessed 28 Aug 2013
Chen Μ, Jin A-J, Wang N, Yu J-K (2006) Synthesis of hydration-resistant CaO refractory by addition of MgO. Dev Chem Eng Miner Process 14(3/4):409–416
Chong C, Specht E (2006) Reaction rate coefficients in decomposition of lumpy limestone of different origin. Thermochim Acta 449(1–2):8–15
Commandré J-M, Salvador S, Nzihou A (2007) Reactivity of laboratory and industrial limes. Trans IChemE A 85(A4):473–480
Criado JM, Ortega A (1992) A study of the influence of particle size on the thermal decomposition of CaCO3 by means of constant rate thermal analysis. Thermochim Acta 195:163–167
Dash S, Kamruddin M, Ajikumar PK, Tyagi AK, Raj B (2000) Nano-crystalline and metastable phase formation in vacuum thermal decomposition of calcium carbonate. Thermochim Acta 363:129–135
Davies PJ, Bubela B (1973) The transformation of nesquehonite into hydromagnesite. Chem Geol 12:289–300
de Souza F, Bragança SR (2013) Thermogravimetric analysis of limestones with different contents of MgO and microstructural characterization in oxy-combustion. Thermochim Acta 561:19–25
Erwing J, Beruto DT, Searcy AW (1979) The nature of CaO produced by calcite powders decomposition in vacuum and in CO2. J Am Ceram Soc 62:580–584
European Lime Association (2014). http://www.eula.eu/. Accessed 15 April 2014
Filippou D, Katiforis N, Papassiopi N, Adam K (1999) On the kinetics of magnesia hydration in magnesium acetate solutions. J Chem Technol Biotechnol 74:322–328
Fuller EL, Yoos TR (1987) Surface properties of limestones and their calcinations products. Langmuir 3:753–760
Ghosh A, Tripathi HS (2012) Sintering behaviour and hydration resistance of reactive dolomite. Ceram Int 38:1315–1318
Glasson DR (1967) Reactivity of lime and related oxides XVI. Sintering of lime. J Appl Chem 17:91–96
Hashimoto H, Komaki E, Hayashi F, Uematsu T (1980) Partial decomposition of dolomite in CO2. J Solid State Chem 33:181–188
Hatakeyama T, Zhenhai L (1998) Handbook of thermal analysis. Wiley, Chichester
Irabien A, Toquero A, Ortiz MI (1989) Kinetic behaviour of non-isothermal lime hydration. Chem Eng J 40:93–99
Kantiranis N, Filippidis A, Christaras B, Tsirambides A, Kassoli-Fournaraki A (2003) The role of organic matter of carbonate rocks in the reactivity of the produced quicklime. Mater Struct 36:135–138
Kougemitrou I, Godelitsas A, Tsabaris C, Stathopoulos V, Papandreou A, Gamaletsos P, Economou G, Papadopoulos D (2011) Characterisation and management of ash produced in the hospital waste incinerator of Athens, Greece. J Hazard Mater 187(1–3):421–432
Lanas J, Alvarez JI (2004) Dolomitic limes: evolution of the slaking process under different conditions. Thermochim Acta 423:1–12
Leontakianakos G, Baziotis I, Profitis E, Chatzitheodoridis E, Tsimas S (2013) Assessment of the quality of calcination of marbles from Thassos Island using Raman Spectroscopy and X-Ray Diffraction. In: Proceedings of the 13th international congress, vol XLVII, Chania, Sep 2013. At Chania, Crete
Levin EM, Robbins CR, McMurdie HF, Reser MK (1964) Phase diagrams for ceramists, vol I. American Ceramics Society, Columbus
Maciejewski M, Ostwald HR (1985) Morphological observations on the thermal decomposition of calcium carbonate. Thermochim Acta 85:39–42
Marinori N, Allevi S, Marchi M, Dapiaggi M (2012) A kinetic study of thermal decomposition of limestone using in situ high temperature X-ray powder diffraction. J Am Ceram Soc 95(8):2491–2498
Matabola KP (2006) The effects of hydration agents on the hydration of industrial magnesium oxide. Master Thesis, University of South Africa
McCauley RA, Johnson LA (1991) Decrepitation and thermal decomposition of dolomite. Thermochim Acta 185:271–282
McIntosh RM, Sharp JH, Wilburn FW (1990) The thermal decomposition of dolomite. Thermochim Acta 165:281–296
Moffat W, Walmsley MRW (2004) Improving energy efficiency of a lime kiln. In: Proceedings of joint SCENZ/FEANZ/SMNZI conference, Hamilton
Moropoulou A, Bakolas A, Aggelakopoulou E (2001) The effects of limestone characteristics and calcination temperature to the reactivity of the quicklime. Cem Concr Res 31:633–639
Oates JAH (1998) Lime and limestone. Chemistry and technology, production and uses. Wiley-VCH, Weinheim
Potgieter JH, Potgieter SS, Moja SJ, Mulaba-Bafubiandi A (2002) An empirical study of factors influencing lime slaking. Part I: production and storage conditions. Miner Eng 15:201–203
Rocha SDF, Mansur MB, Ciminelli VST (2004) Kinetics and mechanistic analysis of caustic magnesia hydration. J Chem Technol Biotechnol 79(8):816–821
Rodriguez-Navarro C, Ruiz-Agudo E, Luque A, Rodriguez-Navarro AB, Ortega-Huertas M (2009) Thermal decomposition of calcite: mechanism of formation and textural evolution of CaO nanocrystals. Am Mineral 94:578–593
Sadykov V, Kharlamova T, Pavlova S, Muzykantov V, Ishchenko A, Krieger T et al (2010) Doped lanthanum silicates with the apatite structure as oxide-ion conducting electrolytes: synthesis, characterization and application for design of intermediate temperature solid oxide fuel cell. In: Borowski M (ed) Perovskites: structure, properties and uses series: chemical engineering methods and technology. Nova Science Publishers, Inc., New York, pp 85–92
Sasaki K, Qiu Χ, Hosomomi Υ, Moriyama S, Hirajima T (2013) Effect of natural dolomite calcination temperature on sorption of borate onto calcined products. Microporous Mesoporous Mater 171:1–8
Sawada Y, Uematsu K, Mizutani N, Kato M (1978) Thermal decomposition of hydromagnesite 4MgCO3–Mg(OH)2–4H2O under different partial pressures of carbon dioxide. Thermochim Acta 27:45–59
Schwarzkopf F (1994) Lime Burning Technology—a manual for lime plant operators, 3rd edn. Svedala Industries Kennedy, Van Saun
Shi H, Zhao Y, Li W (2002) Effects of temperature on the hydration characteristics of free lime. Cem Concr Res 32:789–793
Siagi ZO, Mbarawa M, Mohamed AR, Lee KT, Dahlan I (2007) The effects of limestone type on the sulphur capture of slaked lime. Fuel 86:2660–2666
Smidt E, Meissl K, Tintner J, Ottner F (2010) Interferences of carbonate quantification in municipal solid waste incinerator bottom ash: evaluation of different methods. Environ Chem Lett 8:217–222
Stathopoulos VN, Ladavos AK, Kolonia KM, Skaribas SP, Petrakis DE, Pomonis PJ (1999) Preparation, characterization and surface acid catalytic activity of microporous clays pillared with Al1–xFe x Oy (x = 0.00 to 1.00) oxidic species. Microporous Mesoporous Mater 31(1–2):111–121
U.S. Department of the Interior (2013) U.S. Geological Survey, mineral commodity summaries. http://minerals.usgs.gov/minerals/pubs/mcs/. Accessed 15 April 2014
U.S. Department of the Interior (2014) U.S. Geological Survey, mineral commodity summaries. http://minerals.usgs.gov/minerals/pubs/mcs/. Accessed 15 April 2014
Wilburn FW, Sharp JH (1993) The bed-depth effect in the thermal decomposition of carbonates. J Therm Anal 40:133–140
Wolter A (2006) Hydration kinetics of quicklime. In: Oral presentation, 11th International Lime Association congress, Prague, 18 May
Yeprem HA, Türedib E, Karagöz S (2003) A quantitative-metallographic study on the sintering behavior of dolomite. Mater Charact 52:331–340
Acknowledgments
Sincere thanks are due to Onassis Foundation for the financial support to G. Leontakianakos. Dr. Laura Carmody is thanked for improving the English grammar and style on an earlier version of the manuscript, and Dr. Paul Asimow for an English edit of the final version.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Leontakianakos, G., Baziotis, I., Papandreou, A. et al. A comparative study of the physicochemical properties of Mg-rich and Ca-rich quicklimes and their effect on reactivity. Mater Struct 48, 3735–3753 (2015). https://doi.org/10.1617/s11527-014-0436-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1617/s11527-014-0436-y