Abstract
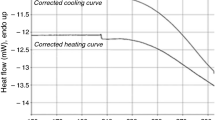
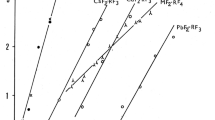
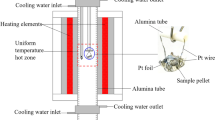
The thermal stability of thaumasite, Ca3Si(OH)6(SO4)(CO3)·12H2O, has been determined and new thermodynamic data defining its stability and solubility are reported. The absolute upper limit of stability of thaumasite in a saturated aqueous solution at 1 bar pressure is 68 ± 5 °C. This numerical value apparently conflicts with the widespread view that thaumasite is only stable at low temperatures, <20 °C. The belief that thaumasite preferably forms at low temperatures is also encouraged by thermodynamic calculations: its free energy of formation enlarges rapidly with decreasing temperatures. Thaumasite solubility increases rapidly with rising temperature so it is destabilised in many mineralogical phase assemblages, often at temperature much below 68 °C. These so called conditional limits, i.e., conditional on thaumasite coexisting in particular mineralogical assemblages, are important in practice to limit its occurrence at high temperatures but should nevertheless not be confused with absolute stability, as determined from the pure compound. Hence two types of stability exist, absolute and conditional. The possibility of stabilising thaumasite to higher temperatures, >68 °C, by forming solid solution with ettringite components is discussed: it remains a theoretical but as yet unproven possibility.











Similar content being viewed by others
Notes
Balonis [3] confirms that rigorous washing of ettringite, synthesised by the sucrose method, reduces or eliminates these DTA/DTG effects. Thus it is likely that sucrose is strongly retained at the surface of thaumasite. However, rigorous washing also alters the bulk composition of the solid owing to its incongruent dissolution.
Except for minor sulfate which is sorbed by C–S–H.
References
Aguilera J, Blanco-Varela MT, Vaszquez T (2001) Procedure of synthesis of thaumasite. Cem Concr Res 31:1163–1168
Atkins M, Glasser FP, Jappy TG, Kindness A, Moroni LP (1991) Effect of elevated temperature on blended cement in radioactive waste disposal. TR-AU-1 HMIP Disposal Assessments, HMIP Commissioned Research
Balonis M (2008) Personal communication
Barnett SJ, Macphee DE, Lachowski EE, Crammond NJ (2002) XRD, EDX and IR analysis of solid solutions between thaumasite and ettringite. Cem Concr Res 32:719–730
Bellmann F (2004) On the formation of thaumasite CaSiO3.CaSO4.CaCO3·15H2O: Part I. Adv Cem Res 16(2):55–60
Bensted J, Prakash Varma S (1974) Studies of thaumasite part II. Silic Indus 39:11–19
Blanco-Varela MT, Aguilera J, Martinez-Ramirez S (2006) Effect of cement C3A content, temperature and storage medium on thaumasite formation in carbonated mortars. Cem Concr Res 36:707–715
Blanco-Varela MT, Rubio F, Aranda MAG, De la Torre AG, Martinez-Ramirez S (2011) Thermal behaviour of thaumasite. In: Proceedings of 13th international congress on the chemistry of cement, Madrid
Brown P, Hooton RD (2002) Ettringite and thaumasite formation in laboratory concretes prepared using sulfate-resisting cements. Cem Concr Compos 24:361–370
Damidot D, Barnett SJ, Glasser FP, Macphee D (2004) Investigation of the CaO-Al2O3-SiO2-CaSO4-CaCO3-H2O system at 25°C by thermodynamic calculations. Adv Cem Res 16(2):69–76
Diamond S (2003) Thaumasite in Orange county, Southern California: an inquiry into the effect of low temperature. Cem Concr Compos 25(8):1161–1164
Edge RA, Taylor HFW (1971) Crystal structure of thaumasite, [Ca3Si(OH)6·12H2O](SO4)(CO3). Acta Crystallogr 27:594–601
Erlin B, Stark DC (1965) Identification and occurrence of thaumasite in concrete. In: Symposium on the effects of aggressive fluids in concrete
Giampaolo C (1986) Dehydration kinetics of thaumasite at ambient pressure. Neues Jahrbuch Mineralogie, Monatshefte 3:126–134
Götz-Neunhöffer F, Neubauer J, Schwesig P (2006) Mineralogical characteristics of ettringites synthesised from solutions and suspensions. Cem Concr Res 36:65–70
Helgeson HC, Delany JM, Nesbitt HW, Bird DK (1978) Summary and critique of the thermodynamic properties of rock-forming minerals. Am J Sci 278A:1–229
Hummel W, Berner U, Curti E, Pearson FJ, Thönen T (2002) Nagra/PSI Chemical Thermodynamic Data Base 01/01. Universal Publishers, Parkland
Kresten P, Berggren G (1976) The thermal decomposition of thaumasite from Mothae kimberlite pipe, Lesotho, Southern Africa. J Therm Anal Calorim 9(1):23–28
Kulik D, Berner U, Curti E (2003) Modelling chemical equilibrium partitioning with the GEMS-PSI code. PSI Scientific Report, vol IV, pp 109–122. http://gems.web.psi.ch
Lothenbach B, Matschei T, Moeschner G, Glasser FP (2008) Thermodynamic modelling of the effect of temperature on the hydration and porosity of Portland cement. Cem Concr Res 38(1):1–18
Macphee, D, S. Diamond (2003) Thaumasite in cementitious materials. Special issue. Cem Concr Compos 805-1209
Macphee D, Barnett S (2004) Solution properties of solids in the ettringite—thaumasite solid solution series. Cem Concr Res 34(9):1591–1598
Matschei T, Lothenbach B, Glasser FP (2007) Thermodynamic properties of Portland cement hydrates in the system CaO-Al2O3-SiO2-CaSO4-CaCO3-H2O. Cem Concr Res 37:1379–1410
Ramachandran R, Gupta PK (1985) An improved spectrophotometric determination of silicate in water based on molybdenum blue. Anal Chim Acta 172:86–89
Schmidt T, Lothenbach B, Romer M, Scrivener K, Rentsch D, Figi R (2008) A thermodynamic and experimental study of the conditions of thaumasite formation. Cem Concr Res 38(3):337–349
Struble LJ (1986) Synthesis and characterisation of ettringite and related phases. In: Proceedings of 8th international congress on the chemistry of cement, Rio de Janeiro, Keine Datum, pp 582–588
Thaumasite Expert Group (1999) The thaumasite form of sulfate attack: risks, diagnosis, remedial works and guidance on new construction. Report of the Thaumasite Expert Group, Department of the Environment, Transport and the Regions, London
Thönen T, Kulik D (2003) Nagra/PSI chemical thermodynamic data base 01/01 for the GEM-Selektor (V.2-PSI) Geochemical Modeling Code: Release 28-02-03. PSI Technical Report TM-44-03-04. http://les.web.psi.ch/Software/GEMS-PSI/doc/pdf/TM-44-03-04-web.pdf
Torres SM, Leal AF, Viera AAP, Barbosa NP (2011) Thaumasite form of sulphate attack in a tropical climate weather. In: Proceedings of 13th international congress on the chemistry of cement, Madrid
van Aardt JHP, Visser S (1975) Thaumasite formation: a cause of deterioration of portland cement and related substances in the presence of sulphates. Cem Concr Res 5(3):225–232
Zhou Q, Glasser FP (2001) Thermal stability and decomposition mechanisms of ettringite at <120°C. Cem Concr Res 31:1333–1339
Acknowledgments
The support of NANOCEM, a European industrial/ academic partnership for fundamental research on cementitious materials, is acknowledged. We also thank EMPA (Switzerland) and in particular, Dr. Barbara Lothenbach, for helpful discussion, work experience and the use of EMPA internal equipment during this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Matschei, T., Glasser, F.P. Thermal stability of thaumasite. Mater Struct 48, 2277–2289 (2015). https://doi.org/10.1617/s11527-014-0309-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1617/s11527-014-0309-4