Abstract
The impact of phthalates, which might be present in a cementitious repository as degradation products of superplasticisers or PVC additives, on the migration behaviour of An(III)/Ln(III) was investigated in batch sorption experiments on hardened cement paste. In the absence of organics, An(III)/Ln(III) sorb strongly on HCP with distribution ratios, Rd, of 105 to 106 L kg−1. Above a no-effect level of ~ 10–3 mol L−1 phthalate, a distinct decrease of the An(III)/Ln(III) sorption was observed with sorption reduction factors (i.e., the ratio between the Rd values without and with organic ligand) of 100 to 1000 at phthalate concentrations of 10–1 mol L−1. This sorption reduction is attributed to the effect of Ca complexation with phthalate on the stability of calcium silicate hydrate (C–S–H) phases as main sorbing phase. These results and ongoing diffusion experiments indicate an increase in the mobility of An(III)/Ln(III) in cementitious barriers with increasing phthalate concentrations.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The safety concept for deep geological disposal of radioactive wastes is based on the confinement of the radioactivity over long periods of time by a multiple barrier system, combining an engineered barrier system and a suitable host-rock formation. Many disposal concepts for radioactive wastes developed internationally make extensive use of cementitious materials, for example, for solidification and stabilisation of low and intermediate level wastes (L/ILW), or as construction materials, drift and shaft seals, and backfill [1,2,3,4]. This is due to the general radionuclide fixation and immobilisation properties of cementitious materials and their low permeability and diffusivity. Although the behaviour of safety-relevant radionuclides in cementitious environments has been investigated extensively in the last decades, the impact of organic degradation products, originating from organic waste components or from superplasticisers in cementitious materials, on the migration of radionuclides under highly alkaline, cementitious conditions is not yet fully understood [5].
Polyvinyl Chloride (PVC) and styrene-based ion-exchange resins (IER) form substantial constituents of L/ILW streams in various European countries such as France, Switzerland, Sweden, and Finland [6]. Thus, the aim of our work, performed within the framework of EURAD WP3 CORI (Cement-Organic-Radionuclide Interaction), was to fill knowledge gaps in the understanding of the impacts of the presence of phthalate species (C8H4O42−; degradation product of superplasticizers used as additives to enhance the workability of concrete [7] or from PVC plasticisers such as di(2-ethylhexyl)phthalate (DEHP) or di-isononyl phthalate (DINP) [8]) and tri-methyl-amine (TMA, C3H9N; degradation product of basic anion exchange resins [9]) on the migration behaviour of safety relevant radionuclides in cementitious barriers. Here, we focus on the potential effect of phthalates on the solubility and sorption of trivalent actinides and lanthanides such as 241Am(III) and 152Eu(III) on hardened cement pastes. Batch sorption experiments were performed to quantify the uptake of these radionuclides in cementitious materials and assess the effects of phthalates on their sorption behaviour. The role and significance of phthalates on the migration behaviour of 241Am and 152Eu in a cementitious repository are further explored in diffusion experiments using cement monoliths.
Materials and methods
Preparation of HCP
The hardened cement paste (HCP) was manufactured by the Commissariat à l'énergie atomique et aux énergies alternatives (CEA) in February 2016 from a CEM V/A cement (CEM V/A (S-V) 42.5N CE PM-ES-CP1 NF, Calcia, Rombas) with a cement/water ratio of 0.40 as described by Macé et al. [10]. The CEM V/A is a ternary blended cement comprising approximately 50 wt% clinker, 25 wt% blast furnace slag and 25 wt% fly ash (mainly silica fume) and is expected to be used in the French nuclear waste disposal program [11, 12]. The main constituents of the cement paste are C–S–H, calcite, ettringite, mullite, portlandite, alite and quartz as well as an amorphous fraction of unreacted slag and fly ash [12]. Further information on the chemical composition of the binder is given in Table S1 in the Supplementary Information. The HCP cylinders were stored in fully saturated conditions at room temperature in a glovebox under controlled atmosphere (Ar) to avoid carbonation. The HCP was mechanically crushed and sieved to < 100 µm for use in batch sorption experiments. For diffusion experiments, the HCP cylinder was sliced with a diamond saw to obtain HCP discs.
Batch sorption and diffusion experiments
Batch sorption studies with both 241Am and 152Eu on CEM V/A HCP were carried out in a glove box under Ar atmosphere (O2 and CO2 levels < 5 ppm) at different phthalate concentrations (phthalic acid, 99.5% Sigma-Aldrich; concentration range 10–1 to 10–5 mol L−1) and in absence of phthalate ions; initial 241Am and 152Eu (Eckert & Ziegler Nuclitec GmbH) concentrations in the experiments were 10–8 to 10–10 mol L−1 and 3.0 × 10–8 to 5.0 × 10–11 mol L−1, respectively. The solution used in the sorption experiments was prepared by immersing crushed CEM V/A HCP in Milli-Q® water (Millipore, 18.2 MΩ cm−1 at 25 °C; total organic carbon (TOC) content 2 ppb) at a solid-to-liquid (S/L) ratio of 0.9 g L−1 as described by Pointeau et al. [13]. After 15 days of equilibration, the artificial cement water (ACW) is filtered and used in sorption experiment to mimic the degradation state III. Sorption experiments were performed with a fixed S/L ratio of 5 × 10–4 kg L−1 prepared in (ultra)centrifuge tubes (polyethylene centrifuge tubes with sealing cap). Before adding aliquots of 241Am and 152Eu, the suspensions were equilibrated for at least 5 months with ACW and—in case of the experiments with phthalate addition—the pH was adjusted to 12.2 using NaOH, where required. The sorption experiments were performed for 7 days, based on literature data on the fast uptake kinetics of An(III)/Ln(III) on cementitious materials [14]. After one week equilibration time, the suspensions were centrifuged (6000 rpm, 1 h) and analysed for 241Am and 152Eu with an ultra-low-level liquid scintillation (LSC) counter Qantulus 1220 (Perkin Elmer, USA) equipped with alpha–beta discrimination option. Samples for the LSC were prepared by mixing aliquots of the supernatants with Ultima Gold LLT LSC cocktail (Revvity). The counting efficiencies of LSC were 82.6% for 152Eu and nearly 100% for 241Am.
The results of the sorption tests were expressed in form of the distribution ratio Rd [L kg−1] as:
where As [Bq kg−1] is the tracer activity sorbed on the solid phase, Al [Bq L−1] the tracer activity in the liquid phase at the end of the experiment, Ai [Bq L−1] the initial tracer activity in solution, V [L] the volume of the liquid phase, and m [kg] the mass of solid phase used in the experiment, respectively. Wall sorption effects and the formation of 241Am and 152Eu colloids were considered in the data evaluation based on the results of solubility tests as described by Tits and Wieland [13].
Diffusion experiments with 152Eu on CEM V/A HCP monoliths were carried out inside an Ar filled glove box in absence and presence of phthalate ions ([phthalate]tot = 10–2 mol L−1), using diffusion cells adapted from the work of Yuan et al. [15]. Cylindrical HCP samples (thickness: 10 ± 0.1 mm; cross-sectional area: 19.6 cm2) were embedded in an epoxy resin (Epofix, Struers GmbH) forming a sample holder. The sample holders were mounted in a cylinder made of polymethylmethacrylate (PMMA), creating an “inlet reservoir” on one side and an “outlet reservoir” on the other. The total volumes of the inlet and outlet reservoirs were 126 mL and 7 mL, respectively. A filtered portlandite-saturated solution, representative of cement degradation state II (pH 12.5; [Ca]tot = 22 mM) is used in the diffusion experiments. After equilibration, diffusion was initiated by spiking the solution inside the inlet reservoir with 152Eu to reach an initial concentration of 2 × 10–9 mol L−1 below the solubility limit in degradation state II. The 152Eu tracer solution used was carrier free. Openings in both reservoirs allow regular sampling of the solutions; the 152Eu activities of the samples were measured by LSC as described above. In addition to 152Eu, the solutions in the inlet reservoir were spiked with HTO in tracer concentrations ([HTO]initial = 5 × 10–10 mol L−1) to determine the effective diffusivity and the accessible porosity of the HCP monoliths for a (assumingly) non-sorbing tracer. After each 2 mL sampling, the solution in the outlet reservoir is fully replaced by a fresh portlandite-equilibrated filtered water. Data evaluation is performed by inverse modelling of the diffusion data using the finite element code COMSOL Multiphysics (Ver. 6.1).
Thermodynamic modelling
To aid interpreting the batch sorption and diffusion experiments in presence and absence of organics, the aqueous speciation of the components in solution and saturation indices of relevant solid phases were calculated using the geochemical codes PhreeqC Ver. 3.5.0 [16] and GeoChemist’s Workbench (GWB) Ver. 11.0.8 [17]. The ThermoChimie v.10d thermodynamic database (Consortium Andra—Ondraf/Niras—RWM [18, 19]) was used for the thermodynamic modelling. The activities of aqueous species were calculated using the specific ion interaction (SIT) approach [20,21,22] in PhreeqC and the B-dot equation [17] in GWB, respectively.
Results and discussion
The results of the 241Am and 152Eu batch sorption experiments with CEM V/A HCP are shown in Fig. 1 and given in Supplementary Material (data publication [23]). In the absence of phthalate, the strong sorption of 241Am and 152Eu on cementitious materials is re illustrated by Rd values in the range 105 L kg−1 < Rd < 106 L kg−1. These results are in good agreement with those obtained for degradation state I and state III in the literature [14, 24,25,26], considering that in absence of organics, no distinct effect of the cement degradation state on An(III)/Ln(III) sorption is observed. Comparison between the Rd values obtained for 241Am and those for 152Eu indicates slightly stronger uptake of 152Eu by the HCP CEM V/A state III for the highest concentrations investigated. The retention of 241Am and 152Eu by cementitious materials is mainly due to uptake by calcium-silicate-hydrates (C–S–H) and is thought to be associated with a two-step process [13, 27]: 241Am and 152Eu form first an inner sphere complex on the C–S–H phases. This is followed by a second step in which 241Am and 152Eu become incorporated into the C–S–H structure, substituting Ca in the C–S–H interlayer and in the Ca-octahedral layer.
Effect of phthalate on the uptake of 241Am and 152Eu by HCP prepared from CEM V/A. The solid line represents the best fit obtained from the data using a Freundlich isotherm. The experimental data of 241Am and 152Eu uptake parameters are in the Supplementary Material (data publication [23])
In the investigated systems, 241Am and 152Eu uptake by HCP CEM V/A was found to be strongly affected by the aqueous phthalate concentration. The results in Fig. 1 show a plateau of the uptake at low phthalate concentrations (< 10–3 mol L−1), where the Rd values are rather similar to those in absence of phthalate, followed by a distinct decrease in the Rd values above a so-called “concentration edge” [28]. Above the “concentration edge”, the Rd values of 241Am and 152Eu decrease by several orders of magnitude to values of about 103 L to 104 L kg−1 at phthalate concentrations of 10–1 mol L−1. These results indicate that the sorption reduction factors (SRF, i.e. the ratio of the Rd value in absence of organics to the one in presence of the organic ligand) for An(III)/Ln(III) are about 100 to 1000 at this phthalate concentration.
At phthalate concentrations of 10–3 mol L−1 and below, Rd values of 241Am are generally somewhat lower than those of 152Eu, suggesting a slight difference in the sorption behaviour of 241Am compared to 152Eu in cementitious materials, with stronger retention of the latter. However, at the highest phthalate concentrations, the sorption behaviour of both radionuclides is rather similar.
Under near neutral conditions, phthalates are strong complexing ligands for An(III)/Ln(III) [7], leading to a strong decrease in the sorption of these radionuclides to clay rocks [29]. However, the geochemical speciation calculations showed that, under cementitious conditions, their impact on the speciation and solubility of 241Am or 152Eu is negligible (cf. Fig. 2). The contributions of aqueous Am- and Eu-phthalate complexes to the total radionuclide concentrations were less than 10–4% even at the highest phthalate addition. Thus, the observed “concentration edge” and the decrease in An(III)/Ln(III) sorption with increasing phthalate concentrations cannot be explained by complexation with the organic compound but rather suggests an effect of the phthalate addition on the cementitious material used in the sorption experiments.
In Fig. 3, the calculated aqueous speciation of Ca in a solution equilibrated with HCP CEM V/A is shown as function of the phthalate concentration. At phthalate concentrations above around 10–3 mol L−1, the contribution of the aqueous Ca-phthalate complex (Ca(C8H4O4)0) to the total Ca concentration increases distinctly, becoming the dominant Ca-complex at phthalate concentrations above about 10–2 mol L−1. The increasing complexation of Ca in solution causes the dissolution/destabilisation of Ca-bearing phases in the cementitious material, leading to decalcification of the C–S–H phases, which are the main sorbing phases for An(III)/Ln(III) in cementitious materials. This destabilisation of C–S–H phases is also indicated by the calculated saturation indices. In the absence of the organic complexant or at low phthalate concentrations (i.e., ≤ 10–3 mol L−1), respectively, the solution equilibrated with HCP CEM V/A is virtually in equilibrium with a C–S–H phase with low Ca/Si ratio (CSH0.8; saturation index SI ≈ 0). With increasing phthalate addition, the saturation index of this phase decreases to about − 0.8 (at 10–1 mol L−1 phthalate), indicating the increasing destabilisation of the C–S–H.
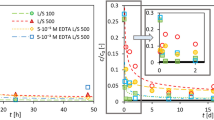
The diffusion experiments and the interpretation of the diffusion data are still ongoing, since the HTO flux has not reached yet the diffusive steady state. In Fig. 4, the concentration of 152Eu in the inlet of the diffusion cells in the absence and presence of phthalate is illustrated as a function of time. Based on the sorption results, an increase in the mobility of An(III)/Ln(III) in cementitious barriers in the presence of high phthalate concentrations is expected. However, the preliminary diffusion data shown here are probably mainly associated with sorption processes occurring at the surface of the monoliths. Even in the presence of phthalate, An(III)/Ln(III) are strongly sorbing radionuclides on cementitious materials (log Rd about 3.5 {L kg−1} at 10–1 mol L−1 phthalate); thus, a break-through of such radionuclides in diffusion experiments may require years. Therefore, the transport parameters of 152Eu will be evaluated by fitting the experimental tracer distribution profiles in the HCP, using laser ablation coupled to ICP-MS for providing the concentration profiles in the HCP disks. Based on initial transport simulations, the diffusion profiles will be determined after 120 days of diffusion.
Conclusions
As expected, in absence of phthalate ions, 241Am and 152Eu sorb strongly on HCP CEM V/A as illustrated by Rd values in the range of 105 to 106 L kg−1. Above a no-effect level of about 10–3 mol L−1 phthalate, the presence of phthalate species leads to a strong decrease in the sorption of An(III)/Ln(III) in this system by several orders of magnitude to Rd values of about 103 to 104 L kg−1, resulting in sorption reduction factors of about 100 to 1000 at 10–1 mol L−1 phthalate. This sorption reduction is not caused by the complexation of An(III)/Ln(III) by the organic ligand under the prevailing highly alkaline conditions. However, it is rather due to the decalcification of C–S–H as the main sorbing phases for An(III)/Ln(III) in consequence of the increasing formation of Ca-phthalate complexes in solution. Further effects might be due to the sorption of phthalate ions by the C–S–H phases. The results indicate that the presence of elevated phthalate concentrations will enhance the mobility of An(III)/Ln(III) in the cementitious barriers of a repository system, even without complex formation with the radionuclides. This effect of phthalates on the migration behaviour of An(III)/Ln(III) will be further evaluated in the ongoing diffusion experiments using HCP monoliths.
Data availability
The data generated during the current study are available at https://doi.org/https://doi.org/10.26165/JUELICH-DATA/PCZCKP.
References
ANDRA, Dossier 2005—Andra Research on the Geological Disposal of High-Level Long-Lived Radioactive Waste—Results and Perspectives. Andra—Agence nationale pour la gestion des déchets radioactifs (Châtenay-Malabry, France, 2005).
G. Kosakowski, U. Berner, E. Wieland, M. Glaus, C. Degueldre, Geochemical Evolution of the L/ILW Near-Field. Nagra Technical Report 14–11 (Nagra, Wettingen, Switzerland, 2014)
Z. Drace, M.I. Ojovan, A summary of IAEA coordinated research project on cementitious materials for radioactive waste management, in Cement-Based Materials for Nuclear Waste Storage. ed. by F. Bart, C. Cau-dit-Coumes, F. Frizon, S. Lorente (Springer, New York, 2013), pp.3–11
C. Jantzen, A. Johnson, D. Read, J. Stegemann, Cements in waste management. Adv. Cem. Res. 22, 225–231 (2010). https://doi.org/10.1680/adcr.2010.22.4.225
M. Altmaier, V. Blin, D. García, P. Henocq, T. Missana, D. Ricard, J. Vandenborre, SOTA on cement-organic-radionuclide interactions. Final version as of 19.05.2021 of deliverable D3.1 of the HORIZON 2020 project EURAD. EC Grant Agreement No: 847593 (2021).
L. Abrahamsen-Mills, J.S. Small, Organic-containing nuclear wastes and national inventories across Europe, in The Microbiology of Nuclear Waste Disposal. ed. by J.R. Lloyd, A. Cherkouk (Elsevier, Amsterdam, 2021), pp.1–20
D. García, M. Grivé, L. Duro, S. Brassinnes, J. de Pablo, The potential role of the degradation products of cement superplasticizers on the mobility of radionuclides. Appl. Geochem. 98, 1–9 (2018). https://doi.org/10.1016/j.apgeochem.2018.09.0047
J. Colombani, G. Herbette, C. Rossi, C. Joussot-Dubien, V. Labed, T. Gilardi, Leaching of plasticized PVC: effect of irradiation. J. Appl. Polym. Sci. 112, 1372–1377 (2009). https://doi.org/10.1002/app.29612
C. Rébufa, A. Traboulsi, V. Labed, N. Dupuy, M. Sergent, Experimental design approach for identification of the factors influencing the y-radiolysis of ion exchange resins. Radiat. Phys. Chem. 106, 223–234 (2015). https://doi.org/10.1016/j.radphyschem.2014.07.020
N. Macé, P. Fichet, S. Savoye, J. Radwan, C. Lim, S. Lefèvre, J. Page, P. Henocq, Use of quantitative digital autoradiography technique to investigate the chlorine-36-labelled radiotracer transport in concrete. Appl. Geochem. 100, 326–334 (2019). https://doi.org/10.1016/j.apgeochem.2018.12.014
O. Bildstein, F. Claret, Stability of clay barriers under chemical perturbations, in Natural and Engineered Clay Barriers. ed. by C. Tournassat, C.I. Steefel, I.C. Bourg, F. Bergaya (Elsevier, Amsterdam, 2015), pp.155–188
F. Claret, S. Grangeon, A. Loschetter, C. Tournassat, W. De Nolf, N. Harker, F. Boulahya, S. Gaboreau, Y. Linard, X. Bourbon, A. Fernandez-Martinez, J. Wright, Deciphering mineralogical changes and carbonation development during hydration and ageing of a consolidated ternary blended cement paste. IUCrJ 5, 150–157 (2018). https://doi.org/10.1107/S205225251701836X
I. Pointeau, C. Landesman, E. Giffaut, P.E. Reiller, Reproducibility of the uptake of U(VI) onto degraded cement pastes and calcium silicate hydrate phases. Radiochim. Acta 92, 645–650 (2004). https://doi.org/10.1524/ract.92.9.645.55008
J. Tits, E. Wieland, Actinide sorption by cementitious materials. PSI Report 18–02, Paul Scherrer (Institut, Villigen, Switzerland, 2018)
T. Yuan, Y. Yang, N. Ait-Mouheb, G. Deissmann, C. Fischer, T. Stumpf, D. Bosbach, A comparative study on heterogeneity of clay rocks using pore-scale diffusion simulations and experiments. J. Geophys. Res. Solid Earth 127, e2022JB025428 (2022). https://doi.org/10.1029/2022JB025428
Parkhurst, D.L., Appelo, C.A.J., Description of input and examples for PHREEQC Version 3—A computer program for speciation, batch-reaction, one-dimensional transport, and inverse geochemical calculations. U.S. Geological Survey Techniques and Methods, Book 6, (2013)
C.M. Bethke, Geochemical and Biogeochemical Reaction Modelling, 2nd edn. (Cambridge University Press, Cambridge, 2008)
E. Giffaut, M. Grivé, Ph. Blanc, Ph. Vieillard, E. Colàs, H. Gailhanou, S. Gaboreau, N. Marty, B. Madé, L. Duro, Andra thermodynamic database for performance assessment: ThermoChimie. Appl. Geochem. 49, 225–236 (2014). https://doi.org/10.1016/j.apgeochem.2014.05.007
M. Grivé, L. Duro, E. Colàs, E. Giffaut, Thermodynamic data selection applied to radionuclides and chemotoxic elements: an overview of the ThermoChimie-TDB. Appl. Geochem. 55, 85–94 (2015). https://doi.org/10.1016/j.apgeochem.2014.12.017
J.N. Brønsted, Studies of solubility: IV. The principle of specific interaction of ions. J. Am. Chem. Soc. 44, 877–898 (1922). https://doi.org/10.1021/ja01426a001
E.A. Guggenheim, The specific thermodynamic properties of aqueous solutions of strong electrolytes. Philos. Mag. A 19, 588–643 (1935). https://doi.org/10.1080/14786443508561403
G. Scatchard, Concentrated solutions of strong electrolytes. Chem. Rev. 19, 309–327 (1936). https://doi.org/10.1021/cr60064a008
N. Ait Mouheb, N. Macé, P. Henocq, D. Bosbach, G. Deissmann, Experimental Data: Effect of Organic Degradation Products on the Migration Behaviour of Radionuclides in Cementitious Materials. https://doi.org/10.26165/JUELICH-DATA/PCZCKP, Jülich DATA, V1
I. Pointeau, C. Landesman, N. Coreau, C. Moisan, P. Reiller, Etude de la rétention chimique des radionucléides Cs(I), Am(III), Zr(IV), Pu(IV), Nb(V), U(VI) et Tc(IV) par les matériaux cimentaires dégradés. CEA report 2004, RT DPC/SECR 03-037 indice A, Commissariat à l’énergie atomique (CEA), Gif-sur-Yvette, France (2004) (confidential report, quoted in [26])
I. Pointeau, B. Piriou, M. Fedoroff, M.-G. Barthes, N. Marmier, F. Fromage, Sorption mechanisms of Eu3+ on CSH phases of hydrated cements. J. Colloid Interface Sci. 236, 252–259 (2001). https://doi.org/10.1006/jcis.2000.7411
M. Ochs, D. Mallants, L. Wang, Radionuclide and Metal Sorption on Cement and Concrete (Springer, Heidelberg, 2016)
J. Tits, T. Stumpf, T. Rabung, E. Wieland, T. Fanghänel, Uptake of Cm(III) and Eu(III) by calcium silicate hydrates: a solution chemistry and time-resolved laser fluorescence spectroscopy study. Environ. Sci. Technol. 37, 3568–3573 (2003). https://doi.org/10.1021/es030020b
J. Tits, E. Wieland, M.H. Bradbury, The effect of isosaccharinic acid and gluconic acid on the retention of Eu(III), Am(III) and Th(IV) by calcite. Appl. Geochem. 20, 2082–2096 (2005). https://doi.org/10.1016/j.apgeochem.2005.07.004
L. Fralova, G. Lefèvre, B. Madé, R. Marsac, E. Thory, R.V.H. Dagnelie, Effect of organic compounds on the retention of radionuclides in clay rocks: mechanisms and specificities of Eu(III), Th(IV), and U(VI). Appl. Geochem. 127, 104859 (2021). https://doi.org/10.1016/j.apgeochem.2020.104859
Acknowledgments
The project leading to this application has received funding from the European Union’s Horizon 2020 research and innovation programme under Grant Agreement No. 847593.
Funding
Open Access funding enabled and organized by Projekt DEAL. Open Access funding enabled by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation)—491111487, and organised by the Projekt DEAL.
Author information
Authors and Affiliations
Contributions
Conceptualization: NAM, DB and GD; data curation: NAM; funding acquisition: DB and GD; investigation: NAM; methodology: NAM, NM and PH; project administration: DB and GD; resources: DB; visualization: NAM; writing—original draft: NAM and GD; writing—review and editing: NAM, NM, PH, DB and GD.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ait-Mouheb, N., Macé, N., Henocq, P. et al. Effects of organic degradation products on the migration behaviour of radionuclides in cementitious materials. MRS Advances 9, 449–455 (2024). https://doi.org/10.1557/s43580-024-00819-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/s43580-024-00819-y