Abstract
Greenhouse gas emission reduction is often cited as a reason for high energy density, next-generation battery development. As lithium-O2 battery research has progressed, researchers have examined the potential of many novel materials in the drive to reduce parasitic reactions and increase capacity. While the field has made great strides towards producing more reliable batteries, there has been little verification that lithium-O2 batteries will reduce net environmental impacts. This paper examines how material selection ultimately impacts lithium-O2 battery environmental impacts. Given that researchers should not wait until lithium-O2 batteries reach commercialization to assess their environmental impact, this paper describes how to incorporate LCA as an integral part of the battery design process. Furthermore, it provides impact factors of many relevant materials to increase the ease of LCA for the field.
Graphic abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The ground transportation and aviation sectors produce a cumulative 30% of global greenhouse gas (GHG) emissions [1]. Legislative targets, such as those imposed by the Code of Federal Regulations in the United States and Regulation (EU) 2019/631 in the European Union have created a driving force for improved electric vehicles. The development of high energy density batteries is projected to increase the driving range of electric vehicles and enable electrified aircraft [2]. Given their high energy density, lithium-oxygen (Li-O2) batteries have received a significant amount of focus; however, commercialization of Li-O2 batteries has been hindered by reaction characteristics that cause capacity fade and a large voltage gap (which leads to poor round-trip energy efficiency). Li-O2 batteries store energy through the reaction of lithium with oxygen to form Li2O2. In a global sense, this can be written as: (Eq. 1) [3].
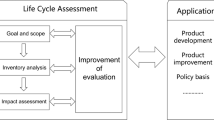
Intermediates, such O2 or LiO2, in the reaction pathway can react with the electrolytes, binders, or carbon materials to form Li2CO2, LiOH, lithium-fluorine compounds, or other unintended side-products, passivating the electrode [3]. To address these degradation pathways, a variety of materials and designs have been explored. Material research generally follows the flow chart in Fig. 1a, where a successful battery design is determined by purely electrochemical characteristics and does not examine the environmental impacts associated with material components or experimental results.
The life cycle impacts of batteries has previously been shown to vary across different battery chemistries depending on both their material components and capacity [2, 4, 5]. Just as life cycle analysis (LCA) is often used as a comparison metric between different battery technologies, it can be applied to inform more holistic materials selection criteria for a single battery chemistry (e.g., Li-O2). This method, proposed in Fig. 1b, can be used to pre-screen materials before their implementation or guide future experiments.
A representative diagram of a current experimental practices for comparison of experimental designs and b a modified experimental design process that includes LCA, highlighted by the pink bubbles, as part of the design process. Within the diagram, ellipses are used to indicate that there are alternatives to the mentioned examples
Materials and methods
This LCA follows framework similar to procedures provided by the ISO 14040 series [6]. The primary goal is to determine the environmental impacts of Li-O2 battery materials and use this information to calculate the impacts of literature-based Li-O2 battery designs. In doing so, the variability in Li-O2 battery impacts are presented across different functional units (FUs).
Throughout this work, there are different scopes for the information presented, corresponding to different FUs. Initially, batteries are compared using cradle-to-gate studies, where the scope includes resource extraction and production, but excludes any emissions related to their use or end-of-life (EOL). In this case, a FU of 1 kg of battery and 1 kWh of battery capacity are compared. For battery studies that included capacity fade data, analysis also includes a cradle-to-grave boundary, where a battery electric vehicle (BEV) is assumed to travel 200,000 km during its lifetime, making the FU 1 km. The cradle-to-grave lifecycle is broken into three parts: production, use, and replacement. The material quantities needed for production were calculated using the energy density and the battery capacity (46.6 kWh) [7].
Equation 2 is used calculate the energy needed to charge the battery during its lifetime:
where T is the total driving distance (200,000 km), \(x_\text{cycle}\) is the cycle driving distance (320 km), \(E_\text{discharged}\) is the battery discharge capacity during use (kWh), \(\eta _\text{charger}\) is the charger efficiency (0.90), and \(\eta _\text{battery}\) the battery’s round-trip efficiency [8]. Replacement is determined by battery degradation, during cycling; the battery needs to be replaced after the efficiency drops to 0.80. [7, 9]
Throughout this study, any impacts related to solvent mixing, cooling systems, and car manufacturing details are excluded. Battery recycling, vehicle accidents, and environmental policy changes are also beyond the this study’s scope. The upstream emissions were determined using the ecoinvent 3.7.1 database [10] the TRACI 2.1 method [11] was used for the life cycle impact assessment (LCIA), and data were processed using openLCA. This work builds on a previous publication, which focused solely on the relationship between battery design and carbon footprint [2]. Here, we expand that work to thoroughly account for the relationship between battery design and a broader range of environmental impacts. The detailed material inventories that support this work are provided in full in the previous paper’s supplementary information.
The five examined battery designs were selected due to their exploration of material impact and sufficiently descriptive methods sections to allow a complete life cycle inventory (LCI). These include: a study examining varying electrolytes, without multiple cycles (abbreviated Electrolyte Var.) [12]; a study examining varied catalysts and varied binders, cycled up to 20 times by (abbreviated Cat. Binder Var.) [13]; a carbon-less, binder-less battery with MoS2 catalyst, and ionic liquid as electrolyte cycled 50 times (abbreviated Carbon/Binder-less) [14]; a similar study, with carbon-less, binder-less battery with MoS2 catalyst, mixed ionic liquid/DMSO as electrolyte, and an anode protective layer, cycled 500 times (abbreviated Anode Protection) [15]; and a battery with a carbon-less, binder-less cathode with gold catalyst, cycled up to 200 times (abbreviated Gold Cat) [16]. The latter three of these five are all variants on the carbon-less, binder-less design, which in this case means no additional carbon materials have been added to the current collector to increase surface area, thus eliminating the need for adding binder. To benefit the broader field, environmental impacts for additional relevant materials have been selected from review papers, as discussed below [17,18,19].
Sensitivity analysis
Sensitivity analysis elucidates how values and assumptions in the LCI affect the overall LCA results. This paper considers two sensitivity analyses:
-
1.
Previous LCAs for Li-O2 batteries have used a single value for the manufacturing energy (MJ per kg of battery fabricated). Energy consumption of a lithium-ion battery can range between 35 MJ kg\(^{-1}_\text{bat}\) to 400 MJ kg\(^{-1}_\text{bat}\) [20]. This relationship is explored throughout the paper using error bars on all graphs.
-
2.
Given an EV’s dependence on grid energy, regional impacts are examined by different grid energy mixes. The baseline case uses a grid mix in China, given their share of battery manufacturing and large size of their BEV fleet. The grids from the United States, Norway, India, and South Africa were also examined. [10].
Life cycle inventory
To determine the environmental impacts of these batteries, the environmental impacts of electrolyte, cathode, and anode materials are first determined. These are shown in Fig. 2, which provides the relative environmental impacts calculated using TRACI 2.1 factors for each material used in the five studies noted above, plus a number of other relevant materials, for comparison’s sake. Figure 2a shows the following binders: lithiated-nafion (Li-Nafion), polytetrafluoroethylene (PTFE), polyvinylidene fluoride (PVDF), polyvinylpyrrolidone (PVP), polyethylene oxide (PEO), and polyethylene (PE). Figure 2b shows carbon paper, nickel foam, aluminum, stainless steel, and copper current collectors Fig. 2c shows the following electrolyte salts or additives: PVDF, LiTFSI, LiClO\(_4\), LiPF\(_6\), LiNO\(_3\), Li, boric acid (BA), tetraethyl orthosilicate (TEOS), and PEO. Figure 2d shows the following solvents: 1-Butyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide (BMIM-C4mim), 1-Ethyl-3-methylimidazolium tetrafluoroborate (EMIM-BF4), Tetraethylene glycol dimethyl ether (TEGDME), dimethyl ether (DME), ethylene carbonate (EC), propylene carbonate (PC), acetonitrile (ACN), and dimethyl sulfoxide (DMSO). Figure 2e shows different processing of lithium anodes. Figure 2f shows platinum, gold, palladium, MoS2, Co\(_3\)O\(_4\), copper phthalocyanine (CoPc), and MnO2. This figure emphasizes that understanding a material’s life cycle impacts is a crucial part of battery design. For example, the catalysts (Fig. 2f) have to be plotted on a log scale, as some catalysts (e.g., pure metals) have significantly higher impacts in almost every category. In the other categories, certain compounds can be identified by their high ozone depletion, likely due to the presence of fluorine in their molecular structure.
If the material is included in a small enough amount or a material provides a substantial increase in capacity or mitigation of capacity fade, the battery itself may have lower environmental impact compared to alternative designs. If this material proves crucial to the function of reliable Li-O2, then efforts should focus on alternative methods for precious metal resourcing, and prospective for those feed-stocks. As an example, it has been shown that gold produced through the recovery of e-waste sees substantial reduction across all impact factors [21]. Examining the allocation of specific contributions to high impact factors can also direct future efforts to reduce a battery’s environmental impact. For example, the nickel based current collector has high amounts of acidification and respiratory effects, which are mostly attributed to sulfur dioxide releases at the mine site. LCA can be used to indicate how feasible it is to improve upstream supply chains, and allow battery researchers to understand their interactions between their work and other fields.
LCI results of commonly used Li-O\(_2\) battery materials: a binders, b current collectors c salts and additives, d solvents, e lithium, and f catalysts. All graphs are normalized to 1.0 for the material identified in the y-axis label. Here, the abbreviations are as follows acidification (acid.), carcinogenics (carc.), ecotoxicity (ecotox), eutrophication (eutroph.), global warming (GHG), non-carcinogenics (non-carc), ozone depletion (ozone dep.), respiratory effects (resp. eff.), smog (smog)
Results and discussion
Figure 3 shows the variation in life cycle environmental impacts for the five mentioned Li-O2 batteries on both the mass and energy basis of the battery. In Fig. 3a, the mass percentage of the batteries is also presented. In this figure, the components of the battery are color coded, while the batteries themselves are differentiated by color intensity. From this mass distribution, we can see that the solvent takes up a large percentage of the battery mass, with the ‘other’ category (oxygen and separator) a close second. Despite this, the oxygen and separator are not distinguished in these graphs, because they do not contribute substantially to the impacts.
Moving to Fig. 3b, we see how battery design affects impact categories. For example, in the case of eutrophication and carcinogens, the lithium is usually responsible for over half of the emissions, indicating that lithium reduction in the cathode could make a significant difference in these categories. Despite the overall poor environmental impacts of gold catalysts in Fig. 2f, the Gold Cat. battery has much lower Ozone Depletion impact than the other battery designs, as it uses a binder-less nickel foam current collector, rather than a binder or carbon paper current collector. This distinction highlights the importance at looking at all the impact factors for design insights.
Examining Fig. 3c, we see that the relative impacts on an energy basis differ from those on a mass basis. This is clearly seen with the Electrolyte Var. and Cat& Binder Var. The Electrolyte Var. has a higher impact factor compared to the Cat & Binder Var. on a mass basis, but not on a energy basis. The energy density of Li-O2 of batteries varies greatly across battery designs. This is further complicated by papers which often reporting energy density on kg\(_{active material}\) basis, rather than a kg\(_{cell}\) basis. As energy density of the entire cell is used to determine the final weight of the battery, and thus the amount of required material for the cradle-to-grave LCA, this is the more relevant metric for LCA impact factors.
a The weight percentages the batteries examined in this study. In this figure, the components of the battery are color coded, while the batteries themselves are in decreasing intensities of these colors. This trend is carried over into b where the relative impact on a mass basis and c energy basis for the five batteries are plotted. From left to right the bars for each type of impact relate to the same batteries as the Mass % graph. The color coding is the same for the different battery components is the same, with added transportation and manufacturing energy. The error bars represent the variation of manufacturing energy, as described in Sect. “Sensitivity analysis”
Sensitivity results
Figure 4a shows the cradle-to-grave impacts for four of the studied batteries. Due to high capacity fade, the Cat. &Binder variation has to be replaced after each use, while the Carbon/binderless battery would be replaced around 6 times, and the Gold Cat around 4 times. In most cases, the low cycling of the Carbon/binderless battery causes it to exceed the environmental impact of other batteries; however looking at the production phase, it has lower impacts. This indicates the trade-off between the two types of batteries, where higher surface area causes higher capacity, but may come at the expense of higher capacity fade. Once again demonstrated by the Gold Cat. battery, low capacity fade does not necessarily mean lower environmental impacts, as the ecotoxicity of this battery is greater. Exploring the relationship between material selection and environmental impacts is crucial for designing next generation batteries. From these results, the authors recommend that high impact materials, such as precious metals, be avoided in battery designs.
Throughout this work, error bars have been used to demonstrate the influence of manufacturing energy (ranging from 35 MJ kg\(^{-1}_\text{bat}\) to 400 MJ kg\(^{-1}_\text{bat}\)), on the LCA. Historically, LCA for Li-O2 batteries has estimated manufacturing energy relative to lithium-ion battery manufacturing. However, given that little information is know about large scale manufacturing of Li-O2 batteries, this may not be a good assumption. As Li-O2 come closer to reaching commercialization, this metric will become critical to determine a battery technology’s sustainability.
a The results of the cradle-to-grave LCA relative to the carbon/binderless battery and b the impact location on the use phase for the carbon/binderless battery relative to a base case using China’s grid. The error bars represent the variation of manufacturing energy, as described in Sect. “Sensitivity analysis”
Figure 4b shows the impact of varying the grid in the context of the carbonless/binderless battery. The use phase, which is represented by the dark red bar, demonstrates which impacts are more sensitive to a specific type of grid energy. In general, the variation in the use phase is high. Grids that have high coal use, such as South Africa, tend to have higher use phase impacts, especially in the case of smog, acidification and eutrophication. In some cases, use phase impacts can exceed production phase. Norway, which has large amounts of hydropower, has much lower emissions attributed to the use phase. This graph also shows the importance of material choice in the future of LiO2 batteries. If capacity fade issues are not resolved the replacement of the battery will eventually negate impact the benefits of a cleaner grid.
Conclusion
There are few papers that address how material selection for Li-O2 batteries changes their environmental impact. The lack of material consistency in the field makes it difficult to determine whether Li-O2 are a worthwhile investment to mitigate climate change and environmental damage. Figure 1 highlights how examination of different battery designs using LCA can be used to guide the battery design process. As researchers develop novel battery materials, they should use LCA to guide material selection and experimental design to achieve the ultimate goal of reducing environmental impacts. Part of this effort is examining environmental impacts over multiple scopes, rather than just GHG emissions, and in conjunction with electrochemical properties. To this end, we make the following recommendations:
-
1.
Methods sections should clearly report information crucial to LCAs, such as thickness of lithium used, electrolyte volume, cathode thickness, and cathode weight percentages.
-
2.
Studies should clearly report energy density, and LCAs should report impact factors based on battery energy density (kWh\(^{-1}\)) on a cell basis, as capacity for batteries varies greatly between experimental designs
-
3.
Researchers should avoid high impact factor materials, such as precious metals
-
4.
LCA related to materials selection in emerging battery designs should include a metric to assess capacity fade
Through the work above, we have shown how LCA can be used to compare materials design strategies across different works, and the importance of using impact factors to investigate pathways for materials research.
Data availability
The data that support the findings of this study are available from ecoinvent but restrictions apply to the availability of these data, which were used under licence for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of ecoinvent.
References
M. Crippa, M. Guizzardi, E. Solazzo, M. Muntean, E. Schaaf, F. Monforti-Ferrario, M. Banja, J. Olivier, G. Grassi, S. Rossi, E. Vignati, GHG emissions of all world countries -, Report, EUR 30831 EN (Publications Office of the European Union, Luxembourg, 2021)
Melodie Chen-Glasser, Amy E. Landis, Steven C. DeCaluwe, Carbon footprint of li-oxygen batteries and the impact of material and structure selection. Journal of Energy Storage 60, 106684 (2023)
J.W. Jung, S.H. Cho, J.S. Nam, I.D. Kim, Current and future cathode materials for non-aqueous li-air (O2) battery technology - a focused review. Energy Storage Mater. 24, 512–528 (2020)
Z. Wu, D. Kong, Comparative life cycle assessment of lithium-ion batteries with lithium metal, silicon nanowire, and graphite anodes. Clean. Technol. Environ. Policy 20, 1233–1244 (2018)
L. Wang, J. Hu, Y. Yu, K. Huang, Y. Hu, Lithium-air, lithium-sulfur, and sodium-ion, which secondary battery category is more environmentally friendly and promising based on footprint family indicators? J. Clean. Prod. 276, 124244 (2020)
International Organization for Standardization. ISO 14040 international standards," in Environmental management-Life Cycle Assessment: Principles and framework, 2006
W. Kempton, Electric vehicles: driving range. Nat. Energy 1, 16131 (2016)
F. Wang, Y. Deng, C. Yuan, Life cycle assessment of lithium oxygen battery for electric vehicles. J. Clean. Prod. 265, 121339 (2020)
P. Marques, R. Garia, L. Kulay, F. Freire, Comparative life cycle assessment of lithium-ion batteries for electric vehicles addressing capacity fade. J. Clean. Prod. 229, 794–797 (2019)
G. Wernet, C. Bauer, B. Steubing, J. Reinhard, E. Moreno-Ruiz, B. Weidema, The ecoinvent database version 3 (part i): overview and methodology. Int. J. Life Cycle Assess. 21(9), 1218–1230 (2016)
J. Bare. Tool for the reduction and assessment of chemical and other environmental impacts (TRACI) version 2.1 user’s guide. US EPA Office of Research and Development, 2014
W. Xu, J. Hu, M.H. Engelhard, S.A. Towne, J.S. Hardy, J. Xiao, J. Feng, M.Y. Hu, J. Zhang, F. Ding, M.E. Gross, J.-G. Zhang, The stability of organic solvents and carbon electrode in nonaqueous Li-O\(_2\) batteries. J. Power Sources 215, 240–247 (2012)
P. Márquez, J. Amici, M.J. Aguirre, F. Herrera, C. Francia, S. Bodoardo, Synergic effect of catalyst/binder in passivation side-products of Li-oxygen cells. J. Solid State Electrochem. 23, 3309–3317 (2019)
M. Asadi, B. Kumar, C. Liu, P. Phillips, P. Yasaei, A. Behranginia, P. Zapol, R.F. Klie, L.A. Curtiss, A. Salehi-Khojin, Cathode based on molybdenum disulfide nanoflakes for lithium-oxygen batteries. ACS Nano 10(2), 2167–2175 (2016)
M. Asadi, B. Sayahpour, P. Abbasi, A.T. Ngo, K. Karis, J.R. Jokisaari, C. Liu, B. Narayanan, M. Gerard, P. Yasaei, A. Mukherjee, K.C. Lau, R.S. Assary, F. Khalili-Araghi, R.R. Klie, C.A. Larry, A. Salehi-Khojin, A lithium-oxygen battery with a long cycle life in an air-like atmosphere. Nature 555, 502–206 (2018)
S. Liu, G. Wang, F. Tu, J. Xie, H.Y. Yang, S. Zhang, T. Zhu, G. Cao, X. Zhao, Au-nanocrystals-decorated \(\delta\)-MnO\(_2\) as an efficient catalytic cathode for high-performance Li-O\(_2\) batteries. Nanoscale 7, 9589–9596 (2015)
Z. Ma, X. Yuan, L. Li, Z.-F. Ma, D.P. Wilkinson, L. Zhang, J. Zhang, A review of cathode materials and structures for rechargeable lithium-air batteries. Energy Environ. Sci. 8, 2144–2198 (2015)
J.-W. Jung, S.-H. Cho, S.N. Jong, I.-D. Kim, Current and future cathode materials for non-aqueous Li-air (O\(_2\)) battery technology - a focused review. Energy Storage Mater. 24, 512–5528 (2020)
J. Lai, Y. Xing, N. Chen, L. Li, F. Wu, R. Chen, Electrolytes for rechargeable lithium-air batteries. Angew. Chem. Int 59(8), 2974–2997 (2020)
J. Dunn, L. Gaines, J.C. Kelly, K. Gallagher, The significance of Li-ion batteries in electric vehicle life-cycle energy and emissions and recycling’s role in its reduction. EES 8, 158–168 (2014)
Z. Li, L.A. Diaz, Z. Yang, H. Jin, T.E. Lister, E. Vahidi, F. Zhao, Comparative life cycle analysis for value recovery of precious metals and rare earth elements from electronic waste. Resour. Conserv. Recycl. 149, 20–30 (2019)
Acknowledgments
Financial support provided by US National Science Foundation, Award 1903440, Program Manager Dr. Raymond Adomaitis.
Funding
Financial support provided by US National Science Foundation, Award 1903440, Program Manager Dr. Raymond Adomaitis.
Author information
Authors and Affiliations
Contributions
MC-G: Conceptualization, Writing—original draft, investigation, data curation AEL: Methodology, Validation, Resources, Writing—review & editing SCD: Validation, Writing—review & editing, supervision.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chen-Glasser, M., Landis, A.E. & DeCaluwe, S.C. Applying life cycle analysis for materials selection in Li-O2 batteries. MRS Advances 8, 803–810 (2023). https://doi.org/10.1557/s43580-023-00610-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/s43580-023-00610-5