Abstract
The study presents bismuth ferrite nanomaterial (BiFeO3) prepared by the green method using Moringa oleifera natural extract as an anode material for supercapacitor application. Cyclic voltammetry, electrochemical impedance spectroscopy, and galvanostatic charge discharge methods were employed to evaluate the electrochemical properties of the nanomaterial at the modified electrode relative to the unmodified electrode. The cyclic voltametric response revealed broad redox peaks and peak separations that clearly indicated the pseudo-capacitive characteristics derived from faradaic reactions. The EIS results indicated that the electrochemical reaction on the material electrode is kinetically and diffusionally controlled. The obtained GCE/BiFeO3 electrode has a specific capacitance of 105 F/g at 0,25 A g−1, an energy density of 90 Wh k/g, and a power density of 0,99 kW k/g. The electrochemical performance revealed an excellent electrochemical response, suggesting that BiFeO3 nanomaterial is an ideal electrode material for supercapacitance applications.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A supercapacitor is a possible alternative that has a higher energy density than a conventional capacitor, fast charge–discharge rates, a higher power density than a battery, a long shelf life, and a high cycle life [1,2,3,4,5]. Recently, binary metal oxides have been the most preferred material as a promising electrode for supercapacitors since they show high electrochemical ion exchange between the electrolyte and electrode material, a large specific surface area, a large pore diameter, and a high volume [1,2,3]. Nanomaterials consist of metal oxide nanoparticles and have been widely used in various applications due to their unique sensing, magnetic, electrical, capacitive, and catalytic properties. Most of them show very good electrochemical performance; hence, they are poisonous to the environment and very expensive; some are inexpensive but have low electrochemical performance. Hence, the search for transition metal oxide with cheap and high electrochemical performance is of great interest [2, 3]. Spine ferrites are the most important types of nanomaterials due to their high chemical stability, large magneto crystalline, and high electrochemical performance. Bismuth ferrite (BiFeO3) is a ferrites nanomaterial that has piqued the interest of researchers due to its magnetic and photocatalytic properties, as well as its electrochemical performance in a wide range of technological applications [6,7,8,9]. It has been widely used in various fields, like Li-ion batteries, photocatalysts, metal-ion removal, supercapacitors, dye degradation, sensors, hyperthermia, etc. Compared to a simple metal, oxide spinel ferrite has better electrochemical activity and greater electronic conductivity [10,11,12,13]. Several researchers have developed different methods to produce bismuth ferrite, such as physical, chemical, and biological methods. Most of the synthetic methods, like physical and chemical methods, have their own advantages and disadvantages. Such as the large quantity of waste generated, high temperatures, high energy consumption, etc [10,11,12,13,14,15]. Therefore, there is still a need to consider developing an easy and economical method for the synthesis of bismuth ferrite nanomaterials and broadening the application scope [6, 7]. Among them all, biological methods are the most promising because of environmentally friendly, simple, inexpensive, low temperature, homogeneity metastable compound, single-step process, no chemical required, production of pure material, no external additives during the reaction, and so on [16,17,18]. When compared to other bio-synthetic routes, the biosynthesis method using natural plant extracts offers advancement because it develops nanomaterials with higher catalytic activity while limiting the use of expensive and toxic chemicals. The natural plant extracts hide some phytochemicals in various parts of plants that act as both capping or stabilization agents and reducing agents in the process of synthesis [18,19,20]. To our best knowledge, this is one of the few report articles that reported on the production of bismuth ferrite nanomaterials using Moringa oleifera plant natural extract and testing their capability as a material for supercapacitors [21]. The published article by Roy et al. showed similar XRD results to this present article; therefore, green synthesis via Moringa oleifera natural extract is suitable to produce bismuth ferrite nanomaterials. [21].
Experimental methods
Materials and methods
The precursor salts (iron III nitrate and bismuth nitrate salts) were purchased from Merck, and Moringa oleifera leaves were purchased from Burkina Faso.
Preparation of a plant extract
30 g of dried Moringa oleifera leaves was immersed in 300 ml of boiled deionized water (DI-H2O) and kept at 50 °C for about 1 h and 45 min. The solution was allowed to cool to room temperature before being drained of all the leaves. The extract was filtered using filter paper and stored at 4 °C for experimental use.
Green synthesis of BiFeO3
About 50 ml of the natural plant extract (50 ml) was used to dissolve 3 g of each of the precursors (bismuth nitrate and iron nitrate). The precursors were dissolved in a natural extract without any addition of heat or chemicals, then covered with foil and kept at room temperature for 18 h. Noticeably, the formation of the bismuth-iron complex was in the form of suspension because no precipitation was observed. The solution was dried at 100 °C in an oven and the remaining powder was collected . Then, the powder was washed several times with distilled water. The powder was annealed in air for 2 h. at 500 °C for further characterization and application.
Characterization
X-ray powder diffraction (XRD) was detected using CuK radiation on a Bruker AXS D8 Focus X-ray diffractometer (λ = 0.15406 nm). Electrochemical performances were conducted on an auto-lab potentiostat (CH Instruments, USA) electrochemical workstation using electrochemical techniques such as cyclic voltammetry (CV), electrochemical impedance spectroscopy (EIS), and galvanostatic charge–discharge (GCD) techniques. The experiments were carried out in a three-electrode system with a glassy carbon working electrode, a platinum wire counter, and an Ag/AgCl reference electrode with a 3 M NaCl salt bridge solution. Voltametric performance was conducted in the potential window from − 0.8 to 0.6 V with scan rates of 50 mV/s and various scan rates of 20–100 mV/s. Impedance performance was measured at a perturbation amplitude of 10 mV within a frequency region of 100 kHz–100 mHz. As an electrolyte, 1 M NaOH was used to measure the electrochemical performance. High-purity argon gas was used to de-oxygenate throughout the electrochemical experiments. Alumina micro-polish (1.0, 0.3, and 0.05 alumina slurries) and polishing pads (Buehler, IL, USA) were used for polishing the electrode surfaces before and after electrochemical measurements.
Fabrication of a GCE/BiFeO3 electrode for the electrochemical studies
About 3 mg of BiFeO3 material was dissolved in distilled water, and Nafion solution was added as a binder. The solution was sonicated for 15 min to form a slurry. About 3 µl of the solution was drop-coated on the surface area of the cleaned glassy carbon electrode, and the electrode was dried in the oven at 35 °C for 1 h. Then rinse it with distilled water and start electrochemical measurements.
Results and discussion
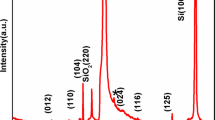
The crystalline structure of the synthesized BiFeO3 nanomaterial was characterized by X-ray diffraction. Figure 1 shows the well-crystallized XRD pattern of the nanomaterial annealed at 500 °C. All the diffraction peaks can be indexed to the rhombohedral phase with space group R3c (no. 166) and lattice parameters a = 5.58 Å and c = 6.93 Å. The XRD pattern indicates the pure structure of BiFeO3 nanomaterial without any indication of impurity phases and agrees with the standard diffraction patterns of rhombohedral JCPD00-014–0181. The characterization peaks of the sample are (101), (012), (021), (202), (211), and (300) crystallographic nucleation planes of the perovskite phase. The strong and high intensity of XRD reflection peaks confirms the good crystallinity of the BiFeO3 nanoparticles. The crystallite size was calculated by the Debye–Scherer formula (Eq. 1) and found to be 20 nm. The lattice parameter was calculated using the reticular plane distance’s relation (Eq. 2) and found to be 5.7948.Å,
where D is the average crystallite size (nm), λ is the x-ray wavelength(15.405 nm), and K is Scherrer’s constant(K¼0.89). β is the full width at half maximum observed in radians and θ is is the angle of diffraction.
The CV voltammogram of bare GCE and GCE/BiFeO3 (modified electrode) at 50 mV/s and different scan rates are shown in Figs. 2A–B. In Fig. 2A (the red curve), GCE/BiFeO3 reveals two redox peaks and a high peak current density. Therefore, the well-defined redox peaks are responsible for the oxidation and reduction of BiFeO3 nanomaterials. The redox reaction peaks are responsible for pseudo-capacitance behavior and are reversible in nature [22, 23]. There is no peak for the bare GCE (the black line); this is an indication that the modified GCE has increased the surface area of the working electrode, which has improved the electron mobility and charge transfer. Figure 2B shows the cyclic voltametric curves at various scan rates ranging from 20 to 100 mV/s. The voltametric curves show that peak current increases with increasing scan rates, indicating that the electrode has excellent rate capability and fast diffusion controlled at the surface [22, 23]. The redox peaks are clearly observed within the potential range, and the curves remain in a quasi-rectangular shape, indicating great reversibility and high electronic conductivity of the nanomaterial-based electrode.
EIS was used to evaluate the kinetic and mass-transport parameters as well as the charge transfer coefficient. The impedance consists of two different plots, i.e., the Nyquist and Bode plots. The first plot is imaginary vs. real impedance at different frequencies, and the second describes absolute impedance vs. logarithmic frequency [24,25,26]. The EIS (Nyquist plot) response of GCE/BiFeO3 electrode was recorded as shown in Fig. 3A. The Nyquist plot in displays a very small semicircle in the high-frequency region that is related to the charge transfer resistance (Rct) and a straight line in the low-frequency region that originates from Warburg impedance. This suggests that electrochemical performance is closely related to the interfacial charge transfer process and diffusion control [23,24,25,26,27].
GCD was performed at current densities of 0.25, 0.50, and 1 A/g and is displayed in Fig. 3B. The charge–discharge curves (GCD curves) had a distorted triangular shape, indicating exceptional electrochemical and pseudo-capacitance behavior. The charge–discharge curves reveal decreasing charge times with increasing current density. Therefore, at high current densities, all the active sites are not accessible for the electrolyte ions to perform the electrochemical reactions. The specific capacitance (Eq. 3), energy density (Eq. 4), and power density (Eq. 5) can be calculated from galvanostatic charge–discharge based on the mass of the three electrodes; the values are demonstrated in Table 1.
td is the discharge time (s, and m is the mass of active material. Id is the discharge current (A), and V is the potential window(V).
Conclusion
The bimetallic compound BiFeO3 was successfully synthesized by the green method using Moringa oleifera natural extract and tested as the electrode material for the supercapacitor. The material reveals a great voltametric response, high electroactivity, and good electrochemical kinetics. Therefore, BiFeO3 nanomaterial is a highly suitable electrode for supercapacitor applications. The voltametric response revealed broad redox peaks and peak separations that clearly indicated the pseudo-capacitive characteristics derived from the faradaic reactions. Charge transfer resistance, or Warburg impedance, was discovered using electrochemical impedance spectroscopy, indicating that the electrochemistry on the surface is kinetically and diffusionally controlled.
Data availability
Data reported in this paper are available upon request to the corresponding author.
References
D.P. Dubal, P. Gomez-Romero, B.R. Sankapal, R. Holze, Nano Energy (2015). https://doi.org/10.1016/j.nanoen.2014.11.013
A. Burke, J. Power Sources 91, 37 (2000)
S.J. Uke, V.P. Akhare, D.R. Bambole, A.B. Bodade, G.N. Front, Mater. (2017). https://doi.org/10.3389/fmats.2017.00021
A. Ghosh, Y.H. Lee, Chemsuschem 5, 480 (2012). https://doi.org/10.1002/cssc.201100645(8
L. Zhang, X.S. Zhao, Chem. Soc. Rev. (2009). https://doi.org/10.1039/b813846j
A. Bismibanu, M. Alagar, J.S. Mercy Jebaselvi, C. Gayathri, IJRASET (2018). https://doi.org/10.22214/ijraset.2018.4299
A. Wrzesińska, A. Khort, M. Witkowski, Sci. Rep. 11, 22746 (2021). https://doi.org/10.1038/s41598-021-01983-z
N.S. Parvathy, R. Govindaraj, Sci. Rep. 12, 4758 (2022). https://doi.org/10.1038/s41598-022-08687-y
I. Sosnowska, T.P. Neumaier, E.J. Steichele, Phys C: Solid State Phys. 15, 4835–4846 (1982). https://doi.org/10.1088/0022-3719/15/23/020
M. Zhu, D. Meng, C. Wang, G. Diao, A.C.S. Appl, Mater. Interf. (2013). https://doi.org/10.1021/am4007353
Y. Ichiyanagi, M. Kubota, S. Moritake, Y. Kanazawa, T. Yamada, T. Uehashi, J. Magn. Mater. (2007). https://doi.org/10.1016/j.jmmm.2006.10.737
W.Q. Meng, F. Li, D.G. Evans, X. Duan, Mater Chem Phys. (2004). https://doi.org/10.1016/j.matchemphys.2004.03.011
H.G. Kim, P.H. Borse, J.S. Jang, E.D. Jeong, O.S. Jung, Y.J. Suh, J.S. Lee, Chem. Commun. (2009). https://doi.org/10.1039/b911805e
Y.L. Liu, Z.M. Liu, Y. Yang, H.F. Yang, G.L. Shen, R.Q. Yu, Sens Actuators B (2005). https://doi.org/10.1016/j.snb.2004.11.026
D. Chen, Y.Z. Zhang, C.J. Tu, Mater. Lett. (2012). https://doi.org/10.1016/j.matlet.2012.05.034
R.K. Gupta, F. Yakuphanoglu, Mater. Lett. (2011). https://doi.org/10.1016/j.matlet.2011.06.091
R.A. Sheldon, J. Mol. Catal. A: Chem. 422, 3–12 (2016)
M. Wieczerzak, J. Namieśnik, B. Kudłak, Environ Int (2016). https://doi.org/10.1016/j.envint.2016.05.017
C.P. Devatha, A.K. Thalla, S.Y. Katte, Axbxh. J Clean. Prod. 139, 1425 (2016)
P.T. Anastas, J.C. Warner, Green Chemistry: Theory and Practice (Oxford University Press, New York, 1998), pp. 29–56
D. Roy, K. Pandey, A.K. Yadav, J. Phys. Sci. (2022). https://doi.org/10.21315/jps2022.33.2.2
V.S. Kumbhar, A.D. Jagadale, N.M. Shinde, C.D. Lokhande, Appl. Surf. Sci. (2012). https://doi.org/10.1016/j.apsusc.2012.06.034
P. Ahuja, R.K. Sharma, G. Singh, J. Mater. Chem. A. (2015). https://doi.org/10.1039/C4TA05865H
R.J. Forster, D. Walsh, K. Adamson, E. Spain, Anal. Sci. 3, 209–217 (2019). https://doi.org/10.15376/biores.16.2.3916-3933
Y. Pan, Y. Zhang, X. Wei, C. Yuan, J. Yin, D. Cao, G. Wang, Electrochim. Acta. 109, 89–94 (2013). https://doi.org/10.1016/j.electacta.2013.07.026
N. Sivakumar, S.R.P. Gnanakana, K. Karthikeyanb, S. Amareshb, W.S. Yoonc, G.J. Park, Y.S. Lee, J. Alloys Compd. 509, 7038 (2011)
S.J. Uke, S.P. Mardikar, D.R. Bambole, Y. Kumar, G.N. Chaudhari, Sci. E. Technol. Mater. (2020). https://doi.org/10.1016/j.mset.2020.02.009
Acknowledgments
This research was generously supported by the UNESCO-UNISA Africa Chair in Nanosciences and Nanotechnology, to whom we are all grateful.
Funding
Open access funding provided by University of South Africa.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Matinise, N., Botha, N., Madiba, I.G. et al. Mixed-phase bismuth ferrite oxide (BiFeO3) nanocomposites by green approach as an efficient electrode material for supercapacitor application. MRS Advances 8, 703–707 (2023). https://doi.org/10.1557/s43580-023-00603-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/s43580-023-00603-4