Abstract
Complexes from catalysts and initiator can be used to insert a specific number of additional chemical functional groups in (co)polymers prepared by ring-opening polymerization (ROP) of lactones. We report on the synthesis of cooligomers from sec-butyl-morpholine-2,5-dione (SBMD) and para-dioxanone (PDX) by ROP with varied feed ratios in the bulk using the catalyst complex SnOct2/2-hydroxyethyl sulfide. Mn of the cooligomers (determined by GPC) decreased with decreasing SBMD feed ratio from 4200 ± 420 to 800 ± 80 g mol−1. When the feed ratio was reduced from 80 to 50 mol% the molar ratio of SBMD of the cooligomers (determined by 1H-NMR) remained nearly unchanged between 81 and 86 mol% and was attributed to a higher reactivity of SBMD. This assumption was confirmed by fractionation of GPC, in which an increase of SBMD with increasing molecular weight was observed. The catalyst/initiator system provides a high potential to create orthogonal building blocks by cleavage of the sulfide bond.
Graphic abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
ROP is well-established in research and industrial production for the synthesis of biodegradable (co)polymers. Tin (II) 2-ethylhexanoate (SnOct2) is a commonly used catalyst for ROP because of its high efficiency. Although soluble tin compounds are in general cytotoxic, the usage of low amounts of this catalyst complies with FDA regulations. However, serious drawbacks of SnOct2 as catalyst are the significant amount of cyclic byproducts and transesterification formed during polymerization as well as the variety of endgroups [1].
We recently reported on the catalyst complex SnOct2/2-hydroxyethyl sulfide (SnOct2/HES) enabling ter-oligodepsipeptides (ter-ODP) with a high content of reactive terminal groups and a significantly lower amount of macrocycles as byproducts when compared with SnOct2 [2]. Another advantage of this catalyst is the possibility to cleave the disulfide group of the synthesized polymers with a reducing agent [2] such as dithiothreitol and thereby generating a bifunctional oligomer carrying a sulfide group on one side and a hydroxyl group on the other as demonstrated earlier [2]. We investigated this catalyst complex for the ROP of a morpholine-2,5-dione (MD) [3,4,5,6,7], PDX and their cooligomers. A series of homo- and cooligomers was synthesized by ROP of SBMD (1) and PDX (2) in one-pot reactions, in which the ratio of SBMD to PDX was varied. The structure of the oligomers was characterized by 1H-NMR. The number average molecular weight (Mn) was calculated from gel permeation chromatography (GPC), 1H-NMR spectroscopy, and MALDI-TOF–MS.
Materials and methods
Chemicals
2-Hydroxyethyl disulfide (99.8%), dibutyl tin(II) oxide (DBTO, 98%) and 4 Å molecular sieves were obtained from Sigma-Aldrich (Steinheim, Germany). SBMD was synthesized according to the method described in reference [6]. p-dioxanone (PDX, purity > 99%) was purchased from Daiwa Kasei Ind. (Japan). 2-Hydroxyethyl disulfide was dried over 4 Å molecular sieves, DBTO was dried in high vacuum. Dimethyl sulfoxide-d6 (DMSO-d6, 99.9%) was purchased from Merck (Darmstadt, Germany).
Methods
Instrumental methods
Instrumental methods have been described in Supplementary Information.
Synthesis methods
The synthesis of SnOct2/HES has been described in reference [2]. In brief, equimolar amounts of 2-hydroxyethyl disulfide and dibutyl tin(II) oxide were refluxed in toluene over molecular sieves at 120 °C for 24 h under inert conditions yielding 72% of SnOct2/HES.
Homo- and cooligomers were polymerized via ROP of the PDX, SBMD and mixtures thereof using the tin(IV) alkoxide (3) as catalyst-initiator complex. The Mn aimed at was 1500 g mol−1. SBMD and PDX were recrystallized and dried over molecular sieve (4 Å) for two days before use. The homo- and cooligomers were synthesized in 25 mL pre-dried Schlenk tubes under nitrogen atmosphere at 135 °C (3 h) under intensive stirring. In detail for Oligo_B50P50, under a counter nitrogen flow 1.5 mmol (0.15 g) PDX and 1.5 mmol (0.25 g) SBMD were placed in a flame dried Schlenk flask equipped with a magnetic stirring bar. 0.2 mmol (0.08 g) of the tin alkoxide catalyst were added. Then the Schlenk flask was placed in the oil bath of 135 °C for 3 h, in which the oligomers precipitated from the monomer feed. The crude products were dissolved in 5 mL chloroform (for the second precipitation in 5 mL dimethylene chloride), precipitated twice in 30 mL of cold diethyl ether, and dried under vacuum at room temperature until constant weights were achieved. The yields after precipitation were in the range of 50 to 80%.
Results and discussion
Cooligomers with varied feed rate were synthesized by ROP. The monomer to catalyst complex (SnOct2/HES) ratio was calculated to obtain a targeted Mn of 1500 g mol−1.The samples were named oligo_BαPβ to indicate the initial molar ratio of the two repeating units, where Bα presents the molar fraction of SBMD and Pβ the molar fraction of PDX (Scheme 1). The molar feed ratio of SBMD was reduced stepwise from 100 to 80 to 65 to 50 and 0%. The received oligomers were purified by two-fold precipitation yielding 50 to 80% oligomer. For selected cooligomers the residual SBMB content could be determined before precipitation by 1H-NMR and ranged around 16% whereas the PDX signals were overlaid and prevented determination.
The obtained oligomers were analyzed by 1H-NMR spectroscopy in DMSO (Fig. 1). In case of the SBMD unit, the multiplett at 1.0–0.7 ppm has been assigned to the methyl groups of the SBMD repeating unit. The methine and methylene groups of the SBMD alkyl chain appear at 2.0–1.12 ppm. The methine proton between the carbonyl and the amide group of the SBMD appears at 4.36 ppm and is followed by the multiplett at 4.73–4.48 ppm. The proton of the amide group appears at 8.3 ppm. The two signals for the two methylene groups of the initiator were assigned to the signal at 3.0 ppm (protons vicinal to the disulfide group) and at 4.28 ppm. In case of the PDX homooligomer this peak is shifted slightly to 4.33 ppm. In case of the PDX repeating unit, the methylene proton vicinal to the carbonyl group appears at 4.16 ppm and the two other methylene protons appear at 4.22 ppm and 3.70 ppm. The signal of the OH terminal group, which was confirmed by D2O exchange, is located at δ = 5.55 ppm (δ = 4.62 ppm for the PDX homooligomer). Its intensity (in comparison to the initiator peak at 3.0 ppm) indicates a quantitative functionalization with hydroxyl end groups, however integration of hydroxyl groups needs to be considered with precaution, especially since a peak at 9.5 ppm, which is sensitive to D2O exchange, is attributed to a carboxylic acid end group.
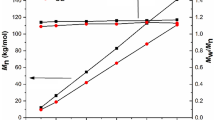
Mn of the oligomers were determined from 1H-NMR spectra (Mn(NMR)) and from GPC (Mn(GPC)). Mn(NMR) was calculated from the equation Mn = M(initiator) + n·M(repeating PDX unit) + n·M(repeating SBMD unit) based on the assumption that one initiator unit was incorporated into each oligomer chain and thereby neglecting other transesterification or initiation by other species. The number of repeating units of the SBMD was calculated from the intensity of the methyl protons in relation to the methylene protons of the initiator unit at 3.0 ppm and the number of PDX repeating units from the intensity of the methylene protons at 4.16 ppm in relation to the initiator unit. Likewise, the molar ratio of SBMD was calculated from the number of repeating units. Interestingly, the molar ratio of SBMD of the cooligomers as determined by 1H-NMR remained nearly unchanged between 81 and 86 mol% despite the fact that the feed ratio was reduced from 80 to 50 mol%. The Mn determined by GPC for the cooligomers decreased with decreasing SBMD feed ratio from 4200 ± 420 to 800 ± 80 g mol−1. The elugrams displayed a monomodal distribution (Supporting Information Figure S1). The polydispersity (Table 1) increased with raising PDX content but was for the homooligomer also 1.3. It can be speculated that SBMD and PDX form aggregates resulting in this increase of Đ. The Mn (MALDI) was determined from MALDI-TOF–MS measurements (Supporting Information Figure S2) based on the series of highest intensity of each sample and varied between 2334 and 1079 g mol−1. In case of the sample Oligo_B100P0 and Oligo_B80P20 the series with highest intensity resembled the SBMD repeating unit including the initiator and OH endgroups. In case of the PDX homooligomer, only the series with highest intensity contained the PDX repeating unit suggesting a ring structure. It is speculated that during the MALDI-TOF MS measurement fragmentation took place, which could explain the low Mn (compared to 1H-NMR and GPC) and the absence of initiator. The mass peaks of the cooligomer Oligo_B65P35 could not be related to chemical structures because of the high number of peak series and because peaks could be associated to different series. The Mn of the homooligomers was 5300 ± 530 g mol−1 (Oligo_B100P0) and 2700 ± 270 g mol−1 (Oligo_B0P100). Mn determined by 1H-NMR were for Oligo_BαPβ with β > 50 lower than the values obtained from GPC and showed a similar decrease of Mn when the SBMD feed ratio decreased. The low achievable PDX molar ratio suggests a more facile polymerization of SMBD compared to PDX. This finding is in line with the observation that synthesis of co-oligomers with a lower SBMD feed rate than 50 mol% did not result in the amounts and molecular weights as expected and maybe be explained by a lower reactivity of PDX in presence of SBMD or the catalyst complex.
In order to investigate a dependency of the SBMD molar ratio of the fractions, Oligo_B80P20 was fractionated by preparative GPC into five fractions. GPC and 1H-NMR investigations showed the expected decrease of Mn in the order of fraction collection from 7200 ± 720 to 900 ± 90 g mol−1 (GPC), which agrees with the Mn determined by NMR evaluation from 3300 ± 720 to 1900 ± 190 g mol−1 considering that for the fractions only standard calibrated GPC was applicable. An exception was Mn (NMR) of Oligo_B80P20-F1 presumably because of the low concentration of oligomer present in this fraction. The SBMD molar ratio decreased from 91 to 74 mol% as determined by 1H-NMR.
Conclusion
Cooligomers of SBMD and PDX were synthesized by ROP in the presence of the catalyst complex SnOct2/HES, which allows thiol functionalization of oligomers after polymerization by reduction of the disulfide bond as demonstrated for ter-oligomers from morpholindiones [2]. Molecular weights as determined by universal calibrated multidetector GPC were for PDX = 50 mol% lower than those determined from 1H-NMR, whereas for SBMD content > 50 mol% higher values were determined by GPC. Molar ratios of the SBMD-PDX differed from the molar ratios of the feed compositions, which may be explained by a lower reactivity of PDX in the presence of SBMD or the catalyst complex. However, the oligomers provided a high degree of hydroxyl end groups as concluded from 1H-NMR spectra. In case of SBMD homo and cooligomers also the presence of carboxylic end groups could be confirmed. MALDI measurements revealed OH end groups for the SBMD homooligomer and Oligo_B80P20. The Mn determined by MALDI are lower than Mn (NMR), which may be explained by fragmentation of the oligomers containing PDX. Future MALDI–TOF–MS investigations are expected to provide more insights for the end group analysis as well in the elucidation of the copolymerization and possible fragmentation behavior.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
H.R. Kricheldorf, S.M. Weidner, High molecular weight poly(l‐lactide) via ring‐opening polymerization with bismuth subsalicylate—the role of cocatalysts. J. Appl. Polym. Sci. 138, 50394 (2020).
X. Liang, M. Behl, A. Lendlein, Dihydroxy terminated teroligomers from morpholine-2,5-diones. Eur. Polym. J. 143, 110189 (2021).
P.J.A. i’nt Veld, P.J. Dijkstra, J.H. van Lochem, J. Feijen, Synthesis of alternating polydepsipeptides by ring-opening polymerization of morpholine-2,5-dione derivatives. Die Makromolekulare Chemie 191, 1813 (1990)
Y. Feng, J. Lu, M. Behl, A. Lendlein, Progress in depsipeptide-based biomaterials. Macromol. Biosci. 10, 1008 (2010)
L. Elomaa, Y. Kang, J.V. Seppälä, Y. Yang, Biodegradable photocrosslinkable poly(depsipeptide-co-ε-caprolactone) for tissue engineering: synthesis, characterization, and In vitro evaluation. J. Polym. Sci. Part A 52, 3307 (2014)
X. Peng, M. Behl, P. Zhang, M. Mazurek-Budzynska, Y. Feng, A. Lendlein, Synthesis of well-defined dihydroxy telechelics by (co)polymerization of morpholine-2,5-diones catalyzed by Sn(IV) alkoxide. Macromol. Biosci. 18, 1800257 (2018)
T.F. Burton, J. Pinaud, O. Giani, Rapid and controlled organocatalyzed ring-opening polymerization of 3S-(Isobutyl)morpholine-2,5-dione and copolymerization with lactide. Macromolecules 53, 6598 (2020)
Acknowledgments
The authors acknowledge gratefully the support of Mr. Olaf Lettau for technical support and GPC measurements and Ms. Susanne Schwanz for MALDI measurements. This work was financially supported by the Helmholtz Association through program-oriented funding and the Helmholtz Graduate School for Macromolecular Bioscience (MacroBio, VH-GS-503).
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Liang, X., Behl, M., Luetzow, K. et al. Cooligomers from morpholine-2,5-dione and para-dioxanone and catalyst complex SnOct2/2-hydroxyethyl sulfide. MRS Advances 6, 764–768 (2021). https://doi.org/10.1557/s43580-021-00082-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/s43580-021-00082-5