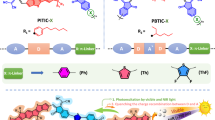
Abstract
The molecularly doped poly(3-hexylthiophene) (P3HT) was used for the first time as a photocathode for reducing oxygen to H2O2. For this purpose, a P3HT film was doped with hexaazatriphenylenehexacarbonitrile, which increased the oxygen reduction current at an applied negative potential in the dark. Visible light illumination of the doped P3HT film significantly facilitated the oxygen reduction with a high current density and shifted the onset potential beyond the reaction equilibrium potential. The oxygen reduction performance of the doped P3HT film is discussed in relation to the energy level diagram.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Polythiophenes are typical organic π-conjugated and conductive polymers. Among the polythiophenes, poly(3-hexylthiophene-2,5-dyil) or poly(3-hexylthiophene) (P3HT) is widely studied for applications in electronic and optoelectronic devices, because of its strong absorption in the visible light range, efficient hole mobility, and tunable highest occupied molecular orbital (HOMO)/lowest unoccupied molecular orbital (LUMO) levels.[1,2,3,4] Metal contamination-free P3HT with high regioregularity and narrow molecular weight distributions are commercially available.[5] The high solubility of P3HT in organic solvents also enables high-quality film formability, facilitating its incorporation during various device fabrication.
Photoelectrodes offer the advantage of converting light energy into chemical reactions, making them a key component in sustainable solutions.[6] Especially, photoelectrochemical cathodes have been extensively investigated to efficiently reduce substrates in water via the reductive reactions of water, carbon dioxide, and nitrogen, using metals, metal oxides, and carbon materials as the catalysts loaded on cathode surfaces.[7] Some of the reactions proceed without an applied electric bias under sunlight illumination, which represents a considerable challenge in sustainable chemistry.[8,9,10,11] However, few studies have been conducted on organic polymer film-based cathodes for the photoelectrochemical reduction reactions, particularly on P3HT film-based ones.[12,13]
Hydrogen peroxide (H2O2) is a metal-free oxidant commonly used in paper and pulp bleaching and wastewater treatment, which is industrially produced via oxygen reduction with hydrogen by the anthraquinone method.[14,15] The production of H2O2 by electrolytic oxygen reduction has also been developed, because pure H2O2 is produced in the separated cathode cell, in addition to achieve its ‛on-site’ production. However, the necessity of designing and constructing electrolytic reactors and high electricity costs are the main challenges related to this technique. The photoelectrochemical oxygen reduction to form H2O2 has been recently reported using the films of polythiophenes as photocathodes,[16,17] because of their robustness in acidic and alkaline electrolyte solutions, strong visible light absorption, reasonable conductivity, and tunable energy levels for the oxygen reduction.
Molecular doping of the π-conjugated polymers has recently attracted considerable attention.[18,19] Doping mechanisms and charge transfer with the strongly electron-withdrawing dopant molecules were intensively studied for various polymers, especially P3HT. Dopant molecules, such as 1,4,5,8,9,11-hexaazatriphenylene hexacarbonitrile (HAT-CN) and fluorinated cyanoquinones, have the ability to regulate the degree of charge separation in P3HT.[20] The P3HT surface can also be easily modified with dopants by coating methods such as spin coating and dipping because the dopant solubility is different from that of P3HT. However, the applications of the molecularly doped π-conjugated polymers have not been examined in detail.
In this paper, capability of the molecularly doped P3HT is demonstrated as a photocathode for the photoelectrochemical reduction of oxygen to H2O2 in an alkaline aqueous solution. We selected regioregular P3HT owing to its strong visible light absorption and hole-transporting capability and HAT-CN was employed as the dopant with a low LUMO level due to six cyano groups and without any absorption in the visible range. The HAT-CN-doped P3HT film was characterized and its photocathode performance was evaluated in addition to touch the H2O2 production via the facile photoelectrochemical oxygen reduction.
Materials and methods
Regioregular P3HT and HAT-CN were purchased from Tokyo Chemical Industry Co. Ltd. (TCI, Japan) and used without further purification. Chlorobenzene and potassium hydroxide (KOH) were procured from Kanto Chemical Co., Inc. An FTO substrate (FTN 1.6, Nippon Sheet Glass) was obtained from Astellatech, Inc.
The FTO substrate was rinsed with isopropanol and a chlorobenzene solution of P3HT was spin coated onto the substrate surface at 2000 rpm for 30 s. The obtained sample was dried and annealed at 120°C for 4 h in air. P3HT doping was performed by dipping the substrate into a 2 mg/mL HAT-CN acetonitrile solution for a few seconds and then dried at 120°C for 10 min.
(Photo)electrochemical responses were recorded by linear sweep voltammetry (LSV), using a potentiostat (HZ-7000, Hokuto Denko) with a 3-electrode-1-compartment setting using Pt as a counter electrode and Ag/AgCl as a reference electrode, respectively. All potentials reported in this work were determined versus the reversible hydrogen electrode (RHE). The active geometrical area of the working electrode was 0.5 × 1.5 = 0.75 cm2. The pH of the KOH aqueous solution as an electrolyte was adjusted to 12 every time before the measurement.
An Asahi Spectra MAX-302 instrument with a visible mirror module was used as the illumination light source with a wavelength range of 385–740 nm wavelength. Bandpass filters (full width at half maximum: ca. 10 nm) were adopted to tune the illumination wavelength for action spectral measurements.
Field-emission scanning electron microscopy was performed at an accelerating voltage of 15 kV using a JEOL JSM-IT800SHL. UV–Vis absorption spectra were recorded with either JASCO V550 or Shimadzu UV-1280 spectrophotometer. X-ray photoelectron spectroscopy profiles were obtained using a PHI VersaProbe II spectrometer (pass energy: 58.7 eV, step size: 0.130 eV/step, Al Kα X-ray source). The HOMO levels of the doped P3HT and P3HT films were estimated by photoelectron spectroscopy in air using an AC-3 photoelectron spectrometer (Riken Keiki, Japan).
Results and discussion
Alkaline solution (pH 12, aqueous KOH) was utilized as the oxygen-containing electrolyte to suppress the side reactions to form hydroperoxy or hydroxyl radicals.[20] Oxygen reduction proceeds according to Eq. (1) to form a hydrogen dioxide anion HO2−:
Here, HO2− is considered equivalent to H2O2 and more robust than H2O2 for side reactions. It reacts sequentially with a proton to form H2O2 under neutral pH conditions. The equilibrium potential for the oxygen reduction reaction (Eeq) at pH 12 is less positive or shallower than that at pH < 11 and is not favorable for the oxygen reduction half reaction, but the counter half reaction of anode, i.e., water oxidation, occurs more easily under such conditions and a bias-free total redox reaction can proceed efficiently using, e.g., inexpensive manganese oxide catalysts for the anodic oxygen evolution reaction in an alkaline with pH 12 solution in the dark.
Linear sweep voltammograms of the HAT-CN-doped P3HT film in the aqueous solution recorded in the dark in air at the pH 12 aqueous solution are shown in Fig. 1, together with those of the P3HT film, the HAT-CN deposited FTO substrate, and the FTO substrate serving as the controls. The reduction current density (J) of the doped P3HT film is ascribed to the oxygen reduction with the negative potential application and is significantly enhanced, while the FTO substrate remained very low. This enhanced oxygen reduction reaction is characterized by two parameters: overpotential (η, the deviation of the reaction onset potential from the equilibrium potential (Eeq) or the excess electrical energy required to progress the electrode reaction) and Tafel slope (inset of Fig. 1, applied potential vs. log J, which reflects the activation energy of an electrode reaction: The smaller the slope is, the faster the reaction kinetics.). The onset potentials of the doped P3HT and the undoped P3HT films were located at more positive (ca. 0.65 V) or smaller η, than that of FTO (ca. 0.5 V) and their Tafel slopes were smaller (ca. − 110 mV/dec) compared to that of FTO (− 150 mV/dec). These results indicate that the P3HT films, as well as the doped one, functioned more efficiently as the cathodes for oxygen reduction.
The photoelectrochemical performance of the doped P3HT and P3HT films are shown in Fig. 2(a). The J values of both films dramatically increased under visible light illumination as compared with those in dark (see Fig. 1). The J of the doped P3HT film reaches a few tens μA/cm2 even under an air atmosphere (its value is dramatically increased by bubbling or supplying more oxygen). As shown in Fig. 2(b), J corresponds to the ON/OFF of light illumination state, which denotes the activity due of the P3HT film.
(a) LSV for the oxygen reduction with the doped P3HT (blue) and the P3HT (red) films under illumination at pH 12, together with the HAT-CN deposited (green) and FTO substrate itself (black), (b) Photoresponse of J at 0.8 V versus RHE, and (c) Action spectrum for the oxygen reduction reaction of the doped P3HT film at 0.8 V versus RHE.
The action spectrum of the photoelectrochemical reaction or the illumination wavelength dependency of J obtained for the doped P3HT film was monitored at an applied voltage of 0.8 V is presented in Fig. 2(c). The J value increases in the visible region corresponding to the absorption spectrum of the doped P3HT film, which indicates that the photoelectrochemical activity of the film is attributed to the light absorption of P3HT and not to the HAT-CN dopant.
Notably, the reaction onset potentials of the P3HT film cathodes, particularly the doped P3HT film, are shifted toward positive values (ca. 1.4 and ca. 1.3 V for the doped and P3HT film, respectively) and beyond the equilibrium reaction potential (Eeq = 0.71 V). This suggests that the oxygen reduction under illumination to produce H2O2 proceeds without any applied bias potential by simply connecting a concurrent water-oxidizing anode in the dark. These J values, onset potentials, and η values of the doped P3HT film are superior to or comparable with those obtained previously in alkaline solutions (Table S1).
The doped P3HT and the P3HT films exhibited brownish colors and visible absorption maximum and edge at ca. 510 and ca. 650 nm, respectively (Fig. S1), which indicated a very efficient visible light absorption capability of the P3HT films based on the developed π conjugation and was not affected by the HAT-CN doping. The optical energy gap of both polymer films estimated from the band edges of the visible absorption spectra was 1.91 eV, which suggested that HAT-CN was located only on the P3HT surface and that the majority of P3HT species remained undoped.
The cross-sectional scanning electron microscopy (SEM) image for the doped P3HT film showed a uniform film formation with a thickness of ca. 300 nm on the FTO substrate [Fig. 3(a)]. The film thickness did not change before and after the HAT-CN doping, indicating that the HAT-CN was located only on the P3HT surface. The obtained SEM images reveal that P3HT covered the rough FTO substrate. The surface of the doped P3HT film [Fig. S2(a)] is slightly rougher than that of the undoped P3HT film [Fig. S2(b)], confirming that the P3HT film surface was modified by the doping of a small amount of HAT-CN.
X-ray photoelectron spectroscopy (XPS) was monitored to estimate the doping amount on the surface of the HAT-CN doped P3HT film [Fig. 3(b, c)]. The binding energies of the S2p and N1s peaks match the previously reported values.[21,22] The molecular ratio or doping degree of HAT-CN of the P3HT monomer unit determined from their area ratio was 1:20, which was reasonable under considering the molecular size of HAT-CN and the doping procedure (dipping in the dopant solution).
The HOMO energy levels of the doped P3HT and P3HT films were measured by photoelectron yield spectroscopy in air (PESA) (Fig. S3) and estimated to be − 5.30 and − 5.10 eV, respectively. It has been reported in literatures[23,24] that the molecular doping of an electron-accepting molecule occurs on a π-conjugated polymer and that the electron at the HOMO of the polymer is partially transferred to the LUMO of the dopant to form a charge transfer complex. The ground-state geometry and HOMO/LUMO levels of HAT-CN and the doped state of quarterthiophene (tetramer, P3HT analog) were obtained by performing density functional theory (DFT) calculation (supplementary Fig. S4) using the B3LYP hybrid functional and 6-31G basis set without considering polarization or diffuse functions on heavy atoms. The HOMO level of the tetramer/HAT-CN complex is − 5.60 eV, which is lower than that of the tetramer alone (− 5.10 eV). This result is in good agreement with the HOMO level of the doped P3HT film estimated with PESA owing to the interaction of the HOMO of the tetramer with the LUMO of HAT-CN (− 4.73 eV).
The mechanism of the photoelectrochemical reaction is illustrated in Fig. 4. When a cathodic bias is applied to the substrate, an electron is injected through the P3HT film into an oxygen molecule in the aqueous solution to form H2O2. The more reactive P3HT film with a smaller Tafel slope is characterized by the smaller bias potential and larger J value in the dark (Fig. 1). Under illumination, the incoming light generates an excited electron, which is injected into an oxygen molecule in the solution. The energy gap between the LUMO level and the equilibrium potential (Eeq) for the oxygen reduction is significantly large, which favors electron injection. The HOMO level of the doped P3HT film (− 5.30 eV) is lower than that of the undoped P3HT film, and the electron injection process to compensate the resided hole is promoted through the hole-transporting P3HT layer to increase J, decrease η, and shift the onset potential toward positive value beyond Eeq.
In this study, the dopant modification of the P3HT film was restricted to its surface, because of the use of the dip coating method. Mixing P3HT and a solution of the dopant solution yielded a black powder containing a charge transfer complex, which may exhibit a higher photoelectrochemical activity for reducing oxygen to H2O2.
Conclusion
A molecularly doped P3HT film was successfully applied to a photocathode to efficiently reduce oxygen to H2O2. The onset potential of the doped P3HT film was more positively shifted with respect to that of the undoped film and exceeded the Eeq value for oxygen reduction, indicating that the oxygen reduction process occurred continuously under illumination without an applied bias potential. Because oxygen is generated by the oxidation of water at the counter anode, H2O2 could be automatically generated in water with the doped P3HT film under illumination. Introducing an anion exchange membrane as a separator[25] would prevent the oxidative side reaction of the hydrogen peroxide migrated toward counter electrode. This cell configuration is now being tested. Molecular doping is not limited to the combination of HAT-CN and P3HT. These findings will be the touchstone for promoting the research toward the decentralized, facile photochemical production of H2O2 from oxygen-containing water.
Data availability
Additional data can be found in the Supplementary Information. Further data are available upon request.
References
J.R. Reynolds, B.C. Thompson, T.A. Skotheim, Conjugated Polymers: Perspective, Theory, and New Materials, 4th edn. (Taylor & Francis Group, Milton Park, 2010)
Z.B. Henson, K. Müllen, G.C. Bazan, Design strategies for organic semiconductors beyond the molecular formula. Nat. Chem. 4, 699–704 (2012). https://doi.org/10.1038/nchem.1422
J. Rivnay, S.C.B. Mannsfeld, C.E. Miller, A. Salleo, M.F. Toney, Quantitative determination of organic semiconductor microstructure from the molecular to device scale. Chem. Rev. 112, 5488–5519 (2012). https://doi.org/10.1021/cr3001109
G. Zhang, F.R. Lin, F. Qi, T. Heumüller, A. Distler, H.-J. Egelhaaf, N. Li, P.C.Y. Chow, C.J. Brabec, A.K.Y. Jen, H.L. Yip, Renewed prospects for organic photovoltaics. Chem. Rev. 122(18), 14180–14274 (2022). https://doi.org/10.1021/acs.chemrev.1c00955
Tokyo Chemical Industry Co. Ltd., Organic Semiconducting Polymer: Highly Regioregular P3HT (2023), https://www.tcichemicals.com/US/en/product/pick/Highly-Regioregular-P3HT. Accessed 21 Nov 2023
H. Shinohara, O. Tsaryova, G. Schnurpfeil, D. Wöhrle, Differently substituted phthalocyanines: comparison of calculated energy levels, singlet oxygen quantum yields, photo-oxidative stabilities, photocatalytic and catalytic activities. J. Photochem. Photobiol. A 15, 50–57 (2006). https://doi.org/10.1016/j.jphotochem.2006.03.024
J. Ma, Photo- and Electro- Catalytic Processes: Water Splitting, N2 Fixing, CO2 Reduction (Wiley-VCH, Hoboken, 2022). https://doi.org/10.1002/9783527830084
H. Nishide, Organic redox polymers as electrochemical energy materials. Green Chem. 24, 465 (2022). https://doi.org/10.1039/D2GC00981A
J. Jayakumar, H.H. Chou, Recent advances in visible-light-driven hydrogen evolution from water using polymer photocatalysts. ChemCatChem 12, 689–704 (2019). https://doi.org/10.1002/cctc.201901725
S. Otep, T. Michinobu, Q. Zhang, Pure organic semiconductor-based photoelectrodes for water splitting. Solar RRL 4, 1900395 (2020). https://doi.org/10.1002/solr.201900395
K. Oka, B. Winther-Jensen, H. Nishide, Organic π-conjugated polymers as photocathode materials for visible-light-enhanced hydrogen and hydrogen peroxide production from water. Adv. Energy Mater. 11, 2003724 (2021). https://doi.org/10.1002/aenm.202003724
R. Wei, M. Gryszel, L. Migliacciobc, E.D. Głowacki, Tuning photoelectrochemical performance of poly(3-hexylthiophene) electrodes via surface structuring. J. Mater. Chem. C 8, 10897–10906 (2020). https://doi.org/10.1039/D0TC01477J
K. Oka, K. Kamimori, B. Winther-Jensen, H. Nishide, Poly(3-alkylthiophene) films as solvent-processable photoelectrocatalysts for efficient oxygen reduction to hydrogen peroxide. Adv. Energy Sust. Res. 2, 2100103 (2021). https://doi.org/10.1002/aesr.202100103
R.L. Myers, The 100 Most Important Chemical Compounds: A Reference Guide (Bloomsbury Publishing, London, 2007)
J.M. Campos-Martin, G. Blanco-Brieva, J.L. Fierro, Hydrogen peroxide synthesis: an outlook beyond the anthraquinone process. Angew. Chem. Int. Ed. 45, 6962–6984 (2006). https://doi.org/10.1002/anie.200503779
W. Fan, B. Zhang, X. Wang, W. Ma, D. Li, Z. Wang, M. Dupuis, J. Shi, S. Liao, C. Li, Efficient hydrogen peroxide synthesis by metal-free polyterthiophene via photoelectrocatalytic dioxygen reduction. Energy Environ. Sci. 13, 238–245 (2020). https://doi.org/10.1039/C9EE02247C
K. Oka, H. Nishide, B. Winther-Jensen, Copolymer of phenylene and thiophene toward a visible-light-driven photocatalytic oxygen reduction to hydrogen peroxide. Adv. Sci. 8, 2003077 (2021). https://doi.org/10.1002/advs.202003077
A.D. Scaccabarozzi, A. Basu, F. Aniés, J. Liu, O. Zapata-Arteaga, R. Warren, Y. Firdaus, M.I. Nugraha, Y. Lin, M. Campoy-Quiles, N. Koch, C. Müller, L. Tsetseris, M. Heeney, T.D. Anthopoulos, Doping approaches for organic semiconductors. Chem. Rev. 122, 4420–4492 (2022). https://doi.org/10.1021/acs.chemrev.1c00581
Y. Yamashita, J. Tsurumi, T. Kurosawa, K. Ueji, Y. Tsuneda, S. Kohno, H. Kempe, S. Kumagai, T. Okamoto, J. Takeya, S. Watanabe, Supramolecular cocrystals built through redox-triggered ion intercalation in π-conjugated polymers. Commun. Mater. 2, 45 (2021). https://doi.org/10.1038/s43246-021-00148-9
C.J. van Velzen, M. Sluyters-Rehbach, J.H. Sluyters, The electrochemical reduction of oxygen to hydrogen peroxide: Part IV. The exclusion of two conceivable mechanisms comprising a dismutation step. J. Electroanal. Chem. Interfacial Electrochem. 200, 291–312 (1986). https://doi.org/10.1016/0022-0728(86)90062-8
F. Guillain, J. Endres, L. Bourgeois, A. Kahn, L. Vignau, G. Wantz, Solution-processed p-dopant as interlayer in polymer solar cells. ACS Appl. Mater. Interfaces 8(14), 9262–9267 (2016). https://doi.org/10.1021/acsami.6b00356
H.J. Jang, J. Wagner, H. Li, Q. Zhang, T. Mukhopadhyaya, H.E. Katz, Analytical platform to characterize dopant solution concentrations, charge carrier densities in films and interfaces, and physical diffusion in polymers utilizing remote field-effect transistors. J. Am. Chem. Soc. 141(12), 4861–4869 (2019). https://doi.org/10.1021/jacs.8b13026
I. Salzmann, G. Heimel, M. Oehzelt, S. Winkler, N. Koch, Molecular electrical doping of organic semiconductors: fundamental mechanisms and emerging dopant design rules. Acc. Chem. Res. 49, 370–378 (2016). https://doi.org/10.1021/acs.accounts.5b00438
I.E. Jacobs, A.J. Moulé, Controlling molecular doping in organic semiconductors. Adv. Mater. 29, 1703063 (2017). https://doi.org/10.1002/adma.201703063
Y. Nara, M. Tanaka, K. Nagasawa, Y. Kuroda, S. Mitsushima, H. Kawakami, Development of highly alkaline stable anion conductive polymers with fluorene backbone for water electrolysis. Polym. Adv. Technol. 33, 2863–2871 (2022). https://doi.org/10.1002/pat.5752
Acknowledgments
This work was partially supported by a Grants-in-Aid for Scientific Research (21H02018) and the Program for Supporting Core Facilities (JPMXS0440500023), from Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan.
Funding
The authors acknowledge the financial support from these fundings: Grants-in-Aid for Scientific Research (21H02018) from Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan. Program for Supporting Core Facilities (JPMXS0440500023) from MEXT, Japan.
Author information
Authors and Affiliations
Contributions
HS contributed to experiment planning, experiment execution, data analysis, and manuscript preparation. HN contributed to experiment planning and manuscript preparation. All authors have given approval to the final version of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shinohara, H., Nishide, H. Molecularly doped polythiophene film as an efficient photocathode for oxygen reduction to hydrogen peroxide. MRS Communications 14, 281–286 (2024). https://doi.org/10.1557/s43579-024-00523-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/s43579-024-00523-w