Abstract
Volatile radioisotopes represent a substantial health risk when released into the environment. To better understand the environmental fate of radioisotopes, the authors constructed a cylindrical steel-walled chamber to simulate the atmospheric processing of volatile radioactive gases. Optical modeling was performed for the properties of simulated sunlight in the chamber to better characterize atmospheric reaction studies. Optical simulations were performed using two wall materials (steel and thin-film silica) and validated against experimental measurements. This optical analysis methodology can be used to improve the fidelity of atmospheric models by accounting for optical inhomogeneities enabling a firmer grasp of radioisotopes’ environmental fate.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Volatile radioisotopes represent a substantial health risk when released into the environment, particularly when present in high concentrations resulting from a nuclear accident. For example iodine-131 presents one of the greatest concerns due to uptake into the thyroid gland.[1] In the natural iodine cycle, iodine is generated as I2 and CH3I in the upper ocean layers. A complex reaction network converts iodine into many chemical forms, including organic iodine compounds and inorganic water-soluble and insoluble compounds. These compounds may exist in the gas or particle phases and partition between them. These complex reaction mechanisms result in large uncertainties in the environmental fate of radioisotopes, such as iodine,[2] and may notably differ from iodine generated in the natural cycle as I2 and CH3I in relatively low concentrations in the upper ocean layers.[3] Historically, the fate of released radioisotopes are studied by dispersion modeling or direct atmospheric measurement.[4,5] Laboratory experiments examining photochemistry and oxidation of organic gases are common outside of radiochemical applications.[6] These experiments are typically conducted in relatively large-scale reactors that are illuminated by simulated sunlight. Such laboratory chamber experiments have not been extensively used to study the chemistry of radioisotopes because these chambers are typically constructed of thin Teflon film. These chambers are incompatible with the study of radioisotopes because Teflon film chambers, which readily absorb iodine, are not robust enough to meet the radiological safety standards of most institutions.
Hidy[6] provides an overview of the necessary features and issues to consider when constructing atmospheric reaction chambers. In addition to minimizing interactions with the container walls, an accurate understanding of the light exposure in the chamber is needed to understand the homogeneity of reactions in the volume. Understanding effective light exposure is critical to translating rates measured in simulated sunlight into those expected in the real world. The chamber discussed in this work was intended for volatile radioisotopes and as such was constructed with safety controls to mitigate the risks associated with operation, ensure the safety of personnel, and prevent accidental environmental release.
To this end, the authors had a cylindrical stainless steel-walled chamber constructed, which met the safety regulations required for radiological work and would provide an environment to simulate the fate of volatile and semivolatile radioisotopes. The chamber had a contained air volume of 1.2 m3. This air volume is considerably smaller than many nonmetal chambers[6] used in atmospheric chemistry, as minimization of the absolute quantity of radioactive material was taken into account. The chamber contained ports for gas injection, gas sampling, pressure and temperate monitoring, and an axis-aligned light pipe (6 in. Conflat® [CF] fused silica window)Footnote 1 where light from a 2.5-kW xenon bulb was introduced into the chamber.
As discussed previously, most atmospheric reaction chambers are constructed of transparent Teflon film, with an optical homogenization zone approximately equal to the volume of the chamber. Such a design does not practically lend itself to radioisotope containment in a robust metallic vessel. Therefore, optical modeling is needed to understand the light intensity throughout the chamber and the impact that inhomogeneous optical exposure will have on photolysis reactions throughout the chamber.
The lamp optics are designed to homogenize the direct light intensity out as much as possible while maintaining a sealed environment. Considering the size of ultraviolet (UV)-transparent windows are limited, the chamber relies on reflections off the chamber walls to further distribute the light and provide irradiance throughout the chamber. The modeling and measurements surrounding the optical irradiation inside the chamber aim to understand the distribution of the light intensity. As the optical modeling is dependent on reflection off the chamber walls, the steel wall geometry and reflection characteristics of the commercial chemical vapor-deposited (CVD) silica have been modeled to understand the influence of the walls on the irradiance inside the chamber. Because photolysis is a primary atmospheric reaction mechanism of many iodine-containing gases, the optical energy that the gas receives must be known to translate results from the laboratory to the real world. A combination of optical modeling (reported in this article) and flow modeling (currently being performed) will be used to calculate the spectrally resolved irradiance of gases throughout the chamber. In this work, the authors describe optical calculations of an atmospheric radioisotope chamber designed to help close the experimental gap between dispersion modeling and direct environmental measurements of volatile radioisotopes through the use of a robust atmospheric chamber.
Materials and Methods
The chamber and port connections were custom made of 304L alloy steel by Kurt J. Leskar, Inc. The chamber is pictured in Fig. 1(a) and the inner volume in Fig. 1(c). The inner surface was lapped to a roughness (Ra) of about 0.6 microns. Twenty-six CF-flanged ports were welded to the circular faces and along the top of the chamber, with a drainage port installed in the bottom. CF ports were chosen for their superior sealing properties and their ability to withstand exposure to the corrosive gases that are likely to be produced from iodine photooxidation. Nonmetal surfaces were minimized and largely limited to valve seals. The circular face ports were installed off-axis to promote gas mixing; the only on-axis port was used to illuminate the chamber air volume. All ports were sealed or plumbed to equipment to provide a leak-tight environment for the introduction of radioactive gases and suspensions.
The chamber was instrumented with two temperature/humidity sensors (Rototronics model HC2-IE302) at opposite ends of the chamber, redundant pressure monitoring (MKS model 722B Baratron and model 910 DualTrans), and gas lines for supply, sampling, and exhaust. All gas lines were valved as close to the interior chamber as possible to minimize excess surface area and dead space in the vessel. The chamber can reach a vacuum of \(<5\times {10}^{-4}\) torr by sole use of a scroll pump (Edwards model nXDS15i). Hydroformed Hydra ~ Cool2 cooling channels piped to a Lauda Variocool VC 3000 chiller with Dynalene HC-30 heat transfer fluid, provide temperature control for the chamber, and were able to maintain a wall temperature between − 5°C and 80°C under the thermal load of the light source and temperatures from − 15°C to 80°C without the light source. This cooling system was necessary because using the light source without any active cooling would lead to wall temperatures exceeding 50°C on the end faces of the chamber. The illumination source was a 2.5-kW xenon XBO lamp (XE2500, SKU: 650-0102)[7] in a custom housing made by Science Tech, Inc. The lamp was attached to the chamber by use of a “light pipe” (10 cm dia., 23 cm long), 6 in. CF flanges, and a fused silica window (Kurt J. Leskar Inc. VPZL-600Q) placed on the center axis of the chamber body. The internal optics of the reflector in the lamp housing were constructed such that the light was focused to the center of the light pipe and at such an angle as to miss the flanges with direct irradiance (a half angle of 23.3°).
To passivate the surface to reduce chemical wall losses and adsorption of reactive compounds as much as possible, a SilcoNert inert amorphous silica-based coating made by CVD was deposited onto the interior surfaces by SilcoTek Corporation (SilcoNert 2000) and capped with a proprietary organic capping agent. As workpieces this large are uncommon, the silica coating thickness was unknown until the chamber was opened and inspected at Pacific Northwest National Laboratory, Fig. 1(b). The modeling effort spanned the anticipated thickness range to provide a model to account for thickness variations in corners as well as variability in the final film that might form if the process were reproduced.
(a) Photograph of the exterior of the chamber prior to sealing of the access ports. (b) A 10-kX UV micrograph of the inside edge of a gasket silica coated with the chamber wall. The cutout shows the silica layer brought out with a 3-pixel Mexican-hat optical filter. (c) A photograph of the inside of the chamber with a steel wall taken prior to silica coating, the photo was taken from the middle access port facing side of the chamber opposite the light pipe. (d) A photograph of the area in the chamber as (c) after silica coating, the photo was taken from the access port seen on the left of (c).
Optical modeling
The optical modeling had the following goals:
-
Seek to understand the effective optical constants and reflectance of the wall materials.
-
Provide a detailed three-dimensional rendering of the inner chamber for illumination calculations.
-
Combine a light source, the obtained reflectance, and the inner chamber to determine the amount of optical energy from the direct illumination and the integral illumination from reflected light by the chamber walls.
The initial optical constant modeling determined the effective optical constants (n and k) using CompleteEASE version 3.65 with the standard optical packages. For the models, the steel chamber wall was assumed to be an infinite absorber, with surface oxide and pure silica layers added onto it. The roughness of the wall was experimentally determined by comparison to a commercial surface finish standard for lapped and polished metals.[8] The effective optical constants were simulated for wavelengths from 200 to 1800 nm and from angles of 1° to 89°.
The resultant optical constant data were averaged over three distinct wavelength regions: UV at 200–400 nm, visible (Vis) at 400–700 nm, and near infrared (NIR) at 700–1800 nm. This averaging helped to gain an understanding of the overall influence of the chamber walls on the chamber illumination. The averaged optical constants were converted to reflectivity by the method in Kasap and Capper.[9] The effective reflectivity for each modeled case was then employed for optical illumination modeling.
Inner volume rendering
AutoCAD version 2017.1.2 (N.402.0.0) was used to create a three-dimensional rendering of the inner chamber volume. The chamber was made to scale, with a 91.5 cm (3 ft) inner diameter and 182.9 cm (6 ft) length, with accurate port placement and sizing (for experimental measurement predictions from the ports). A reflector panel was placed at the end of the light pipe in lieu of a lamp body. The chamber was then rendered to the maximum feature resolution quality (MINRES = 10) and exported to AGI-32 for illumination modeling.
Illumination modeling
The results of the previous two modeling efforts were loaded into AGI-32 version 18.3.2, for illumination analysis. The first step in the modeling was to import the three-dimensional rendering of the chamber. The rendering was then sliced down the center axis for internal investigation and if needed, a perfect mirror was used to cover the central axis of symmetry. Each individual wall face from the ports/caps to the steel chamber wall, and the light pipes were given the applicable reflectance for the simulation based on the results of the optical constant modeling. The rendering mesh level was set to 3.2, ~ 8.8e6 mesh elements. With the three-dimensional rendering loaded with the correct reflectance, a light source was added to the model.
The illumination source was modeled with a point source placed at the center of the light pipe along the center axis and given the same emission angles as the optics of the XBO lamp in its housing. Measurements of the lamp intensity (taken with a Newport 818-UV sensor) were used to inform the illumination source, along with standardization of intensity to conform to Michael.[10] With the light source and chamber characterized and meshed, simulations were run under varying the reflectivity characteristics of the chamber wall’s coating.
Characterization tools and parameters
A power radiometer was used to measure the intensity of the light and verify the model results. The measurements were done with a Newport 818-UV and associated single channel data collection and recorded with PMManager version 3.31. Optical power intensities were calculated from the detector by means of vendor-supplied calibration curves. The mean and standard deviation were collected at each measurement point. The power intensities from the radiometer were also accompanied by spectral measurements.
The light spectra within the chamber were recorded with an Ocean Optics Flame model UV–Visible grating spectrometer.[11] A 10-ft, 1-mm-diameter patch cord with SMA ends and a collimating lens (Ocean Insights 74-VIS, set at 0.5 cm from lens) were used to collect the light.[12] The data were recorded with OceanView version 2.0.7 with an integration time of 6–100 ms (to maximize the signal), 10 averaged spectra, and a box car averaging set to 0.
Measurement conditions
Both the power and spectrum were recorded with the illumination source at 100 percent power (2.5 kW). The lamp had an electrode post that cast a small shadow in the chamber, which was not accounted for in the modeling. During the optical measurements, 10 standard liters per minute of clean air were piped into the chamber to maintain a clean environment and to minimize any external dust coming into the chamber.
Results
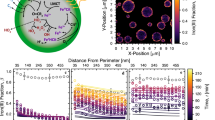
The maximum optical power measured at the fused silica window of the light pipe was 646 ± 6 W. The chamber’s cross-section was 0.65 m2, making the average power density by cross-section 985 ± 8 W/m2, which corresponds well to the current solar standards[10,13] of 875–1050 W/m2. A comparison of the lamp’s power spectrum to solar spectra at air mass (AM) 0–1.5 [10.13] can be seen in Fig. 2(a).
(a) The spectrum of the xenon lamp compared to the spectra for air mass (AM) 0–1.5. (b) Direct intensity guidelines showing optical intensity in the chamber air mass. (c) The refractive index of the inner surface of the chamber wall from a silica thickness of 0 (steel) to 325 nm. The data are simulated from 0° to 90° by steps of 5°. (d) Example of optical intensity modeling results as a function of silica wall coating thickness (rows) and light wavelength (columns).
The results of the direct illumination of the air mass calculation can be seen in Fig. 2(b). The direct illumination is reflected based on the optical properties of the chamber walls (effective n, < n >) as shown in Fig. 2(c). The combination of the direct illumination and reflected intensity allows for the calculation of the optical intensity within the chamber.
Figure 2(d) presents a summary of the reflected light intensity as a function of wall coating and wavelength range. The results are ordered on the vertical axis by the thickness of the silica coating from 0 to 325 nm and on the horizontal by wavelength range (UV = 200–400 nm, Vis = 400–700 nm, NIR = 700–1800 nm).
The optical power intensity was measured before and after the SilcoNert coating was applied to the chamber walls. The optical intensity measured at each port and inside the air mass appeared to vary by about 5 percent. This was attributed to vibration in the gantry built to hold the sensor and lines as seen in intensity variations during short time scales depending on the configuration of the sensor support structure. In Fig. 3(a) and (b), select intensities at the chamber wall and within the chamber volume were reported. The values next to ports were taken at the mouth of the chamber air mass, the large blocks indicate the wall intensities integrated to account for all the light reflected into the sensor (i.e., measurements with the sensor orientated toward the far wall), and the arrowed values report the maximum intensity measured directly in the air mass itself with the arrow signaling the direction of the sensor face. Fig. 3(a) and (b) reports the intensity measurements as the percent of the maximum measured intensity and are for the steel wall and silica-coated wall, respectively.
(a) Compiled intensity power measurements of the light at various points in the chamber before the walls were coated with silica. All values are relativized to the maximum intensity measured at the inlet silica window, of 646 ± 6 W/m2. (b) Compiled intensity measurements of the light in the chamber after SilcoNert coating of the inner surfaces. (c) Average chamber light source spectra for different chamber wall materials.
Figure 3(c) is a comparison of the spectra of the chamber before and after the coating with silica. The light in both figures was introduced from the right of the image through the light pipe. Both represent the average spectrum taken from the same measurement locations within the chamber. The results were averaged to represent an overall spectrum of the chamber.
Discussion
The energetic modeling performed as part of this work was done in the program AGI-32 and was completed by ray tracing from the light source(s) and tallying the amount of energy incident on and reflected from surfaces. Details on the importance of elements and mesh sizes are presented by the company at https://docs.agi32.com/AGi32/Content/calculate/Calculation_Concepts.htm. The models can be verified with radiosity measurements (optical spectrum and intensity), which was completed and a summary is presented in Fig. 2.
This work involved the description of the light intensity within the air volume of the atmospheric radioisotope chamber. Previous works[14,15,16,17] reported on exhaustive research on how to best integrate the models described in Fig. 3(a) and (b) for gases and fine particles and how to treat the intensity at any ports or fine features of the atmospheric radioisotope chamber.
As the light source was introduced at one end of the chamber with a focal length much shorter than the length of the chamber, the largest portion of the optical irradiance came from direct intensity of the lamp. The second portion came from the intensity reflected off any surface opposing the direct intensity of the lamp (i.e., the opposing wall of the chamber) and lastly by integration of intensity perpendicular to the axis of the chamber (i.e., the cylindrical wall of the chamber). According to Elloumi et al.,[17] integration of these light sources will enable direct calculation of the irradiance at any finite point within the chamber. As the conditions for the light intensity will change with experimental conditions (e.g., fine particles scattering the light versus gas-only chemistry), the exact integration method may change, but the results in Fig. 2(a) and (c) can be used to calculate the wall reflection component of the light intensity.
Figure 2(b) and (d) represents the results of such modeling for a case in which the chamber is filled with clean air to demonstrate the utility of the model. The intensities reported in Fig. 2(b) and (d) are the average for each individual mesh element. These intensities are equivalent to the configuration factors in Elloumi et al.,[17] whose summation accounting for angle, distance, and air absorption factors allowed for integration of the light intensity for any given point in the internal air volume.
Figure 3(a) and (b) corroborates the model results presented in Fig. 2(b) and (d). The measured light intensity matches the model within the given error of the measurement for each wall section as well as each port. For a clean air case, the results of the model concurred with the experimental measurements.
Figure 2(c) further corroborates the modeling efforts. The calculation of the effective optical constant (< n >) in Fig. 2(c) shows that for most angles, SilcoNert coating of the chamber wall enhanced the light intensity in the 300–400 nm and 500–600 nm regions if the average silica thickness ranges were 200–250 nm. The slight enhancement of visible light from 630 to 800 nm is indicative of films from 235 to 325 nm. Figure 1(b) shows a UV micrograph of the silica layer in which the thickness varies from 200 to about 350 nm. The experimental measurements corroborate the optical modeling and correlate to the measured intensity enhancement upon silica coating the chamber wall.
Conclusion
Optical modeling provides the data necessary to evaluate the total optical radiation incident on chamber gases using models of the optical intensity inside an optical chamber at any given point. In experiments where optical irradiance is a rate-limiting factor for photocatalytic reactions, this model will clearly enable a means to improve the interpretation of experimental results and assist in the translation of experimental data into an atmospheric model. Direct measurements of optical intensity are well corroborated by optical modeling, providing validation of the optical model. Further analysis of chamber characteristics including wall reactivity (wall losses) and flow modeling is ongoing and will be reported in future work.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Notes
Conflat is a registered trademark of Agilent Technologies, Inc.
References
E. Ostroumova, A. Rozhko, M. Hatch, K. Furukawa, O. Polyanskaya, R.J. McConnell, E. Nadyrov, S. Petrenko, G. Romanov, V. Yauseyenka, V. Drozdovitch, V. Minenko, A. Prokopovich, I. Savasteeva, L.B. Zablotska, K. Mabuchi, A.V. Brenner, Environ. Health Perspect. 121, 865–871 (2013). https://doi.org/10.1289/ehp.1205783
R. Sommariva, W.J. Bloss, R. Von Glasow, Atmos. Environ. 57, 219–232 (2012). https://doi.org/10.1016/j.atmosenv.2012.04.032
A. Saiz-Lopez, J.M.C. Plane, A.R. Baker, L.J. Carpenter, R. Von Glasow, J.C.G. Martín, G. McFiggans, R.W. Saunders, Chem. Rev. 112, 1773–1804 (2012). https://doi.org/10.1021/cr200029u
E.M. Becker, M.J. Myjak, A.M. Prinke, W.J. Kernan, S.G. Homann, J. Environ. Radioact. 228, 106527 (2021). https://doi.org/10.1016/j.jenvrad.2020.106527
Y.H. Koo, Y.S. Yang, K.W. Song, Prog. Nucl. Energy 74, 61–70 (2014). https://doi.org/10.1016/j.pnucene.2014.02.013
G.M. Hidy, Atmosphere 10, 401 (2019). https://doi.org/10.3390/atmos10070401
Osram, XBO Xenon short-arc lamps without reflector. (Osram Entertainment and Industry, 2023). https://www.osram.com/ecat/XBO%20Xenon%20short-arc%20lamps%20without%20reflector-XBO%20Xenon%20short-arc%20lamps-Discharge%20lamps-Industry-Specialty%20Lighting/com/en/GPS01_1028545/. Accessed 21 Jan 2023
Destiny Tools, Understanding Surface Finish.(Destiny Tool, 2023). https://www.destinytool.com/surface-finish.html. Accessed 21 Jan 2023
S. Kasap, P. Capper (eds.), Springer Handbook of Electronic and Photonic Materials, 2nd edn. (Springer, New York, 2017). https://doi.org/10.1007/978-3-319-48933-9
P. Michael, P. A Conversion Guide: Solar Irradiance and Lux Illuminance. (IEEE Datasets 2019). https://doi.org/10.21227/mxr7-p365.
Ocean Optics. Flame Miniature Spectrometer User Manual. Document 225-00000-000-11-201604. (Halma Company, Dunedin, FL, 2015). https://www.manualslib.com/manual/1439052/Ocean-Optics-Flame-S.html
Ocean Insight. Why Collimating Lenses Are So Important, Use Collimators to Align Light in Your Spectrometer Setup. https://www.oceaninsight.com/products/sampling-accessories/free-space-optics/collimating-lenses/collimating-lenses/. Accessed 21 Jan 2023
R. Hulstrom, R. Bird, C. Riordan, Spectral solar irradiance data sets for selected terrestrial conditions. Sol. Cells 15, 365–391 (1985). https://doi.org/10.1016/0379-6787(85)90052-3
V. Pareek, S. Chong, M. Tade, A.A. Adesina, Asia-Pac. J. Chem. Eng. 3, 171–201 (2008). https://doi.org/10.1002/apj.129
R.L. Romero, O.M. Alfano, A.E. Cassano, Ind. Eng. Chem. Res. 36, 3094–3109 (1997). https://doi.org/10.1021/ie960664a
Q. Huang, T. Liu, J. Yang, L. Yao, L. Gao, Chem. Eng. Sci. 66, 3930–3940 (2011)
H. Elloumi, M. Stambouli, J.J. Damelincourt, B. Mrabet, J. Quant. Spectrosc. Radiat. Transf. 74, 731–744 (2002). https://doi.org/10.1016/S0022-4073(01)00282-5
Acknowledgments
This research was supported by the Chemical Dynamics Initiative (CDI) at Pacific Northwest National Laboratory (PNNL). PNNL draws on signature capabilities in chemistry, earth sciences, and data analytics to advance scientific discovery and create solutions to the nation’s toughest challenges in energy resiliency and national security. PNNL is operated by Battelle for the U.S. Department of Energy (DOE) under Contract No. DE-AC05-76RL0-1830.
Funding
Funding was provided by Pacific Northwest National Laboratory.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hubbard, L., Ritzmann, A., Wahl, J. et al. Optical modeling of a radioisotope atmospheric chamber. MRS Communications 13, 256–262 (2023). https://doi.org/10.1557/s43579-023-00337-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/s43579-023-00337-2