Abstract
Rapid testing, generally refers to the paper-based diagnostic platform known as “lateral flow assay” (LFA), has emerged as a critical asset to the containment of coronavirus disease 2019 (COVID-19) around the world. LFA technology stands out amongst peer platforms due to its cost-effective design, user-friendly interface, and low sample-to-readout times. This article aims to introduce its design, use, and practicality for the purpose of diagnosing SARS-CoV-2 infection. A connection is made from the normal COVID-19 immune response to the design and efficacy of rapid testing. Interference in test results is a challenge shared by most diagnostic platforms and can be rooted in various underlying issues. The current knowledge and situation about interference in rapid COVID-19 tests due to variant strains as well as vaccination are discussed. The cost and societal impact are reviewed as they play important roles in determining how to properly implement public testing practices. Perspectives on improving the performance, especially detection sensitivity, of LFA for COVID-19 are provided.
Graphical abstract

Similar content being viewed by others
Introduction
The coronavirus disease 2019 (COVID-19) has, in some way, impacted the lives of most people on Earth and has redefined the world we live in today. Since the identification of SARS-CoV-2, the virus that causes COVID-19, in early 2020,[1] the virus had expanded to more than 200 countries/territories as of July 2021, infecting over 190 million people with a worldwide death toll exceeding 4 million.[2] To give a perspective comparison, this is equivalent to ~ 10% of the death toll of the HIV/AIDS epidemic in under 5% of the time.[3] However, grim the statistics, humankind has come together through research and development to produce over 300 diagnostic platforms qualifying for emergency use authorization by the U.S. Food and Drug Administration (FDA).[4] This combined with vaccinations and public health practices are arguably the most critical efforts to slowing the spread of the virus.
More people now than any time in history require diagnostics with over 450 million COVID-19 tests having been performed in the United States alone as of May 2021 (see Fig. 1).[5] This strain on the healthcare system would not be possible if the only platforms to exist took in the order of hours or days to return results. Reliable and speedy testing is pivotal for the control and management of the COVID-19 pandemic. A rapid test generally refers to the paper-based diagnostic platform known as “lateral flow assay” (LFA, which is sometimes called “test strip”), a well-known example is an over-the-counter pregnancy test. LFAs present many benefits over commonly used “gold standard” polymerase chain reaction (PCR) tests or enzyme-linked immunosorbent assays (ELISA).[6,7] LFAs are handheld, cheap to produce, have fast readout times, and in some cases do not require skilled technicians to operate them.[8]
Chart showing the number of recorded COVID-19 diagnostic tests performed across several representative countries. Data obtained from Our World in Data.[5]
As of July 2021, 44 LFAs from various manufactures have been granted emergency use authorization (EUA) by the FDA for COVID-19 in-vitro diagnostics (IVDs).[9] Representative examples of LFAs are listed in Table I. LFAs are lightweight, compact systems that consist of a paper-based analytical membrane housed inside of a protective casing. LFAs can easily be shipped almost anywhere, allowing countries with shortages due to outbreaks or primitive technology to receive diagnostic technology quickly. Many cities around the world have adapted mobile testing locations where individuals can submit their samples for testing receiving a lab result over the course of a few days. This relies on a cold-chain transport system that delivers the samples from the patient to the testing facility in order to preserve fragile biomolecules. Long shelf lives of LFAs owed to the stability of biomolecule components within the test allow a point-of-care diagnosis that circumvents costs associated with a cold-chain delivery system required for PCR or ELISA.[10,11] These distinctive features of LFA make them particularly suitable for diagnosing COVID-19, especially serological and antigen tests (see Fig. 2).
Distribution of COVID-19 diagnostic platforms possessing EUA by the U.S. FDA categorized by molecular (genetic material), antigen, and serology tests. RT-PCR reverse transcription-polymerase chain reaction, LFA lateral flow assay, ELISA enzyme-linked immunosorbent assays. Data obtained from tables of EUA’s posted by the U.S. FDA, and updated as of 07/20/2021.[49,111,112]
It is worth mentioning that LFAs have displayed usefulness in situations where many people need testing quickly, ideally in the safety of their own homes such as allowing access for air travel and routine, company subsidized employee testing. LFA technology also benefits those living in “testing deserts” where access to testing is limited due to lack of reliable transportation or costly laboratories. Overall implementation of rapid testing for COVID-19 is pivotal to meet the ever increasing demand of society.
Lateral flow assay (LFA) of COVID-19: materials and theory
Detection principle
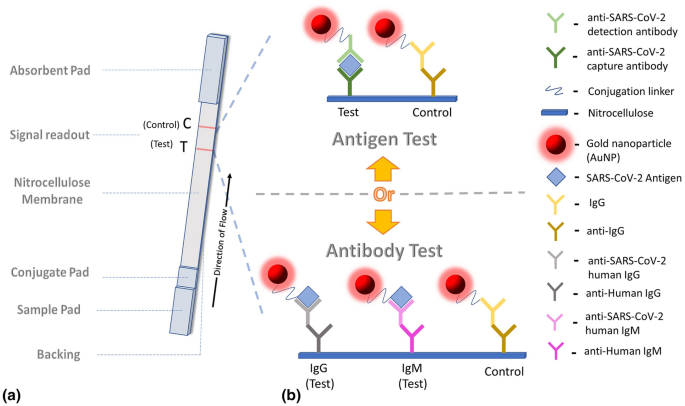
The detection principle of LFA for diagnosing COVID-19 is shown in Fig. 3. The sample is pretreated (if necessary) and the analytical sample is transferred to the sample pad of a test strip. The sample begins to diffuse through the conjugate pad, where it picks up gold nanoparticles (AuNPs) biomolecule conjugates and other necessary additives (Fig. 3(a)). AuNP conjugates in a positive sample will bind to their target analyte, either being an antigen or antibody (serological). The mixture begins to flow through the nitrocellulose until the analyte-AuNP binds to antibodies anchored to the membrane in lines termed test (T) and control (C). The collection of stuck AuNPs generates a color signal that can be seen as an appearing line at either the test or control readouts.

reproduced from Ref [23] with permission from the Royal Society of Chemistry.
Scanning electron microscope image of transversal cut of a nitrocellulose membrane for LFA (Hi-Flow Plus 75, Millipore Corporation). Adapted and
As shown in Fig. 3(b), LFA can diagnose either COVID-19 antigens (i.e., SARS-CoV-2) or antibodies. LFAs that looks for the presence of SARS-CoV-2 antigens will have antibody-AuNP conjugates able to bind the antigen in sample via complex combinations of antigen-specific bonding patterns formed by complimentary epitopes.[12] On the opposite side, if the goal is to detect antibodies produced by a patient in response to an infection of SARS-CoV-2, it would be necessary to have antigen-AuNP conjugates within the conjugate pad. Herein, antigen is typically referring to the SARS-CoV-2 spike (S), nucleocapsid protein (N), and/or fragments of each protein known as its receptor binding domain (RBD).[13] More details about antigen and antibody testing are provided in Sects. “Biomolecules Used in LFA” and “Immune Response to SARS-CoV-2 and LFA Antibody/Antigen Testing” below.
Materials for assembly of LFA
In the design of an LFA there are 6 main components (see Fig. 3(a)): a backing for support, sample pad, conjugate pad, nitrocellulose membrane containing anchored antibodies, absorbent pad, and biomolecule conjugated gold nanoparticles (AuNPs). All these components are typically assembled into a plastic housing.
-
(i)
Backing is the framework that holds the test together, gives reinforcement to other fragile components especially the nitrocellulose membrane, and provides ease in manufacturing. It is composed of three major elements: the semi-rigid plastic coat, adhesives, and liners. The plastic coat is generally polyvinyl chloride (PVC), polystyrene, or polyester and is fabricated with a thickness in the range of 0.005–0.015 inch.[14] Diagnostic grade adhesives are required for lamination of the nitrocellulose and various pads to the plastic support, as more general adhesives can interfere with the function of the product.[14] Pressure sensitive acrylic adhesives are frequently observed in LFA design, binding simply upon applying pressure. For components needing to be laminated onto the backing, the adhesive is usually covered with thin film liners chemically treated with silicone for easy removal during manufacturing.[10,14]
-
(ii)
Sample pad is the first point of contact the analytical sample has with the LFA device and ensures only the desired sample flows onto the nitrocellulose at a controlled rate. It consists of cellulose, glass fibers, or a mixture that can act as a filter passing only the fluidic components of the sample. Glass fibers have intrinsically low bed volumes and high tensile strength allowing for a material that is easy to handle for fabrication and efficiently delivers sample down the strip. However, lower bed volumes make glass less effective for absorbing buffers or detergents during pretreatment.[15] Conversely, cellulose has high bed volumes and low tensile strength making it a fragile material that retains sample; however, they are excellent candidates for pretreatment.[15,16]
The sample pad can act as a primary filter to remove unwanted contaminants. This is especially helpful for samples with high particle load, viscosity, or colored species, which could interfere with flow or signal readout. Many LFAs specific for COVID-19 can use whole blood as the patient sample (see Table 1, for examples), the sample pad functions as a filter for the red blood cells while letting serum pass to the conjugate pad. Tests that use other sample types, such as extracted nasal swab samples, could potentially require different sample pad material to normalize viscosity for optimum lateral flow.[11]
Buffers are often loaded to sample pad to promote elution of the sample down the strip as well as adjust the pH if necessary. Many LFAs rely on buffers to adequate binding of antibodies to their target antigens/antibodies, which happens ideally near biological pH. Samples from various places of the body may have slight variations in the pH at which protein components are most ideal for antibody binding. For example, a normal blood sample’s pH lies in the range of 7.35–7.45,[17] whereas anterior nasal fluid is generally near 6.4.[18] It is ideal that a buffer be at the pH which maximizes the binding equilibrium constant for the desired antibody-antigen capture complex. This is especially important considering this value has been found to be up to 100-fold lower when outside of the ideal range.[19] Other common additives to the sample pad include blocking agents, detergents, and surfactants which are usually proprietary from manufacturers and are screened for compatibility in LFA design.[11,20]
-
(iii)
Conjugate pad is laminated overlapping the sample pad, such that the sample can smoothly transition into it. This pad consists of primarily cellulose to aid in pretreatment with biomolecules conjugated AuNPs (which will be discussed in detail later), but also could be composed of glass fibers or surface modified polyester.[21] These biomolecules are either antigens, in the case of SARS-CoV-2: the spike (S), nucleocapsid (N), or their respective receptor binding domains (RBD), but could also be antibodies specific for the preceding antigens.
-
(iv)
Nitrocellulose (NC) membrane is the analytical membrane where biologically significant reactions take place ultimately leading to signal generation. In a typical design, capture antibodies specific to analytes in sample and biomolecules on AuNPs, respectively, are immobilized in the test (T) and control (C) line regions of a NC membrane.
Despite NC’s critical role, it is notorious for being the most challenging part of the LFA to manufacture consistently.[22] NC is a polymer produced by treating refined cellulose with nitric acid, of which product is dissolved in a mixture of solvents and surfactants termed the “liquor.” The liquor is then precipitated slowly onto a moving belt where environmental conditions are manipulated to remove solvents. The microvariations in this casting process can be seen in the relatively wide range of pore sizes within the final material, varying anywhere from several to tens micrometers (Fig. 4).[22,23] During polymerization, substitution of cellulose hydroxyl groups for nitro groups causes the surface of the final material to bear a negative charge at neutral, or basic pH. This promotes electrostatic interaction between permanent dipoles in proteins, giving NC superior adsorption properties useful for immobilizing capture antibodies.[24] The thickness of the nitrocellulose and pore sizes determine the overall performance of NC as an analytical membrane. Layer thickness determines the volume of sample necessary to perform the test generally varying between ~100 and 150 µm.[22] The pore size determines the capillary flow rate, the rate at which particles move through the membrane, and determines the time required for signal generation.[22,25] A larger pore size will increase the velocity of sample, and provide quicker readout times however, a high flow rate also can decrease the time AuNP conjugates have to bind to their targets. This may result in a lower concentration of captured analyte at the test line, reducing sensitivity.
-
(v)
Absorbent pad is the very last contact that the sample makes with the device. It is responsible for absorbing excess sample and to prevent backflow. Cellulose is the preferred material for this application owed to its high bed volumes.[26]
-
(vi)
AuNP conjugates are arguably the most important unit of LFA design because they are: the signal generating species; the analyte-interacting capturing body; and perhaps the most highly engineerable constituent of the technology.
Gold nanoparticles (AuNPs) with an overall spherical shape and sizes of ~ 10–100 nm are typically used as colorimetric labels in LFAs for COVID-19. These AuNPs have been known to possess the unique optical phenomenon of plasmonic resonance, by which individual particle’s electrons are driven into oscillation by lights electromagnetic field.[27] This causes AuNPs to strongly absorb light at certain wavelengths, giving rise to a red or purple color.[28] As an example, Fig. 5 shows transmission electron microscope image of ~ 40 nm AuNPs and a photograph of the NPs suspended in water.[29]

reproduced from Ref [29] with permission from the Copyright 2017 American Chemical Society (ACS).
Transmission electron microscope image of gold nanoparticles (AuNPs) with an average diameter of ~ 40 nm. Inset shows a photograph of a vial containing aqueous suspension of the AuNPs. Adapted and
The process of AuNP conjugation for COVID-19 LFAs can take place via covalent coupling reactions such as EDC/NHS cross-linking.[30,31] where a carboxyl functional group on the surface of an AuNP is crosslinked to amino acid residues of the desired SARS-CoV-2 antibody or antigen resulting in a stable amide bond between the species.[32] Alternatively, antibody or antigen can be passively adsorbed to the AuNP surface by exploiting Van-der-Waals forces and/or electrostatic interaction.[33,34] This non-covalent method is believed to be sensitive to pH changes and surface chemistry, which may decrease the affinity for conjugation.[34]
Biomolecules used in LFA
The antibody (Ab) array and antigens used to immobilize the analyte of interest depends on both the type of LFA and the desired analyte to be captured (see Fig. 3(b)). Abs used in lateral flow are desired to meet the criteria of being able to maintain conformation after AuNP conjugation and to function smoothly upon rehydration with the sample matrix. LFA Abs are typically used in concentrations tens to hundreds times that of ELISA.[35] Because of this, contaminants pose a heightened risk of increasing non-specific binding and therefore a high level of purification is required before use.[35]
COVID-19 LFAs commonly use monoclonal antibodies (mAbs) at the test line and for conjugation. mAbs are antibodies produced from a single B Lymphocyte cell (e.g., through hybridoma technology[36]) and generally recognize only one epitope on its specific antigen.[37] mAbs are relatively reproducible, providing consistent specificity towards capturing an antigen in a complex matrix, reducing background noise caused by non-specific binding. In contrast, polyclonal Abs are capable of binding to several epitopes on an antigen and contain larger variations between batches. For this reason, polyclonal Abs are not commonly used in COVID-19 LFA and can be considered a risk for manufacture and test performance due to the risk of decreased specificity.[37,38]
-
(i)
In the AuNP conjugates Selection of the biomolecule conjugated on AuNPs is dependent on the desired biomarker to be captured. In an antigen test, it necessary to have a signal transducing antibody capable of forming a selective match with the analyte of interest. It is not necessary for this Ab to be derived from human sources, as long as the conjugate and capture Abs have a binding domain capable of matching the antigen at the same time. For example, in GenBody Inc.’s Covid-19 Ag test, the desired capture antigen is the N protein, therefore in the conjugate pad lies AuNP-conjugated mouse anti-SARS-Cov-2 N protein Abs.[39] In a serological test the order is reversed, meaning the AuNPs are conjugated to antigens specific for the Abs desired to be detected. In Assure’s Covid-19 IgG/IgM Rapid Test Device the conjugate pad contains conjugated recombinant S and N protein, which will bind in the presence of their respective serum Abs.[40]
-
(ii)
In the control line region A visual signal at the control line indicates the validity of the LFA, and therefore demands a signal transducing AuNP-Ab conjugate to bind its capture Ab irreversibly and with high affinity. This is especially important for lateral flow technology where the antibody pair may be in proximity for an estimated 1 to 6 s compared to multi-hour incubation times in ELISA.[35] Commercially available products often utilize Abs derived from mouse, rabbit, or goat hosts. These Abs are required to have one complementary binding domain “anti-” to the other. For instance, in the Cellex q-SARS -CoV-2 IgG/IgM antibody test goat anti-rabbit IgG is fixed to the nitrocellulose membrane which intends to bind AuNP-conjugated rabbit IgG.[41] In another format, the control line originally appears blue upon initiation of lateral flow, an AuNP conjugate will replace the blue colored complex at the control line, indicating a valid test result. This type of test validation is employed by platforms such as Abbott’s Binax Now Covid-19 Ag Card and Healgen’s COVID-19 IgG/IgM Rapid Test Cassette.[42]
-
(iii)
In the test line region At the test line are immobilized capture Abs which intend to bind biomolecule complexes within the sample. Antigen LFAs commonly contain antigen-specific Abs at the test line, which capture the AuNP-Ab conjugate-antigen complex present in positive samples downstream of the conjugate pad (See Fig. 3(b)). Serological LFAs need to bind to Abs naturally occurring in patients and therefore must include an anti-human binding domain. For instance, Assure’s Covid-19 IgG/IgM Rapid Test Device employs anti-human IgG and IgM in its two test lines.[40]
Manufacturing processes
The manufacturing of LFA test strips can be done by hand on small equipment, or by fully automated “all-in-one” machinery such as the Bio Dot RR4510© Reel to Reel Dispensing System. First, capture antibodies are anchored to the nitrocellulose (NC) membrane to form T and C lines by a syringe pump connected to a flexible hollow dispenser. The nature of the interaction between immunoglobulin and NC can be electrostatic, hydrophobic, and/or via hydrogen bonds.[43] The tip can either drag the antibody solution across the strip, as seen in the Bio Dot Frontline© technology, or can spray the solution as a fine aerosol. After pretreatment of the NC, the conjugate and sample pad are pretreated, generally by airjet technology, with the desired conjugates, buffers, detergents, and other essential components finished by drying in a forced air convection oven.[44]
Following the pretreatments, assembly of the LFA test strips begins with a sheet or roll of NC being laminated to the backing, usually performed on a laminating machine such as the Matrix 7100 Reel-to-card Laminator©. Liners on the conjugate and absorbent pad are laminated onto the NC. The sample pad liner is removed and laminated overlapping the conjugate pad. The sheet of NC is then carefully cut using a precision guillotine (e.g., a Biotron CM5000 Guillotine Cutter©) into individual test strips. Finally, the individual strips are fixed into plastic or card housing which indicates the instructions and positions of T and C lines.
Immune response to SARS-CoV-2 and LFA antibody/antigen testing
Upon exposure of SARS-CoV-2 to mucous membranes, the virions use a membrane spike protein to enter healthy respiratory epithelial cells through angiotensin converting enzyme-2 (ACE2).[45] Major Histocompatibility Complexes 1 and 2 bind viral proteins to the surface of infected cells, presenting them to T lymphocytes which differentiate into either effector cytotoxic T cells capable of neutralizing the presenting cell, or helper T cells.[46,47] Activated helper T cells stimulate the production of B cells producing immunoglobulins, including IgM and IgG, to assist in acute and long-lasting Ab mediated immune responses, respectively.[48]
COVID-19 specific IgG and/or IgM immunoglobulins are target biomarkers for serological LFAs (i.e., antibody testing) granted emergency use authorization by the FDA.[49] As such, it is critical to know the timeline at which these biomarkers can expect to be detected in various bodily fluids. Serum IgM is the first serum antibody to be produced in response to SARS-CoV-2 infection. IgM could be detected in as little as 3 days post infection and this antibody persists at peak concentrations only during the acute phase of disease, roughly 2 weeks.[50] IgM is an ideal biomarker to detect a recent infection, especially in the absence of an IgG positive. IgM is followed by the production of IgG,[50] whose peak concentrations can persist up to ~ 45 days following infection, and after which levels steadily decline.[48,50,51] Testing positive for COVID specific IgG indicates a past infection, and seroconversion from an IgG seropositive state to seronegative was found to occur in a significant portion of individuals.[51] This means it is possible for a person to have been infected, yet be under the threshold for many diagnostic tests.
Proteins from the SARS-CoV-2 virion can also be detected (i.e., antigen testing) in serum by exploiting their binding affinity to manufactured antibodies. The viral nucleocapsid phosphoprotein (N) of SARS-CoV-2 packages the viral genome and plays a role in viral self-assembly.[52] The spike protein (S) on the outer membrane surface of the virion uses its receptor binding domain for the host’s ACE2 receptor to penetrate and invade host cells.[53] Viral protein antigens were found to be detectable in blood at peak concentrations in as little as just 5 days following infection which reduce as the production of IgG increases.[54] This presents a target for even earlier detection of active infection.
Taken together, these invasive virions and our adaptive immune responses allow for two LFA testing modes: antigen testing, which points to an active infection and will not show a previously infected individual; and antibody (serological) testing, which can confirm a previous infection for a given time.
Rapid testing and the vaccine
Many people may ask the question: “Will a rapid test tell me if my COVID vaccine has worked?”. The answer is generally no, complex, and dependent on several factors including vaccine choice, the rapid test used, and the individual’s unique serological response.
Two of the widely used vaccines (Moderna and Pfizer-BioNTech) induce immunity by introducing mRNA that tricks the body to endogenously produce viral spike proteins.[55,56] These proteins can be utilized by B lymphocytes to produce IgG antibodies capable of neutralizing active virions. Moderna’s mRNA vaccine targets the production of antibodies towards the receptor binding domain (RBD), a small portion of the spike protein and therefore may cause interference in tests that include S protein RBD AuNP conjugates, such as Assure’s Covid-19 IgG/IgM Rapid Test Device.[40] The Pfizer-BioNTech vaccine does encode for a full-length spike protein and many other S protein fragments including the RBD.[57] This has the theoretical possibility to cause complement binding for the spike protein antibody, however vaccine promoted antibody titers vary between subjects as well as through time, with some subject not having any detectable levels at all post injection.[58,59] Therefore, is unclear in any one case whether a rapid antibody test will have sufficient sensitivity to yield a positive result and is currently the subject of investigation.[60,61] Janssen, the vaccine produced by Johnson & Johnson is composed of a whole inactivated, replication incompetent viral vector called an “adenovirus”.[62] The adenovirus houses the genetic material necessary for the body to produce its own antibody array with Abs specific for many different viral epitopes.[63] Any Abs with specificity for mAbs employed in the test design may react and interfere in the same manner as the Pfizer-BioNTech vaccine.
Rapid antigen tests may also possess cross reactivity with vaccine produced proteins. Vaccine-induced S proteins and RBDs have been shown to be detectable in serum using Moderna’s mRNA vaccine as early as 1 day and up to an average of 15 days following administration.[64] This raises the possibility of false positive results in tests that utilize a full S protein and RBD fragments for individuals receiving Moderna’s and Pfizer-Bio-N-Tech’s vaccines, respectively, within 15 days. Characterization of serum antigen concentrations following Janssen administration are undocumented and it is currently unclear whether this vaccine is capable of interacting with rapid antigen tests.
All vaccines currently authorized produce antibodies specific to either the S protein or its receptor binding domain, making N proteins and their antibodies excellent biomarkers to distinguish a natural infection.[65] One such platform the ADVAITE, RapCov Rapid COVID-19 Test confirms the presence of anti-N IgG, on the downside this test is only able to detect past infections.[66] Currently LFA rapid tests are not sensitive enough to recommend use determining vaccine efficacy, despite the potential benefit to the community and vaccine developers.
COVID variant and its impact on LFA testing
The coronavirus genus is known to have a relatively high rate of mutation.[67,68] Since the introduction of the original strain in 2019, COVID has evolved many defined lineages, with 11 having been found to have heightened risk factors such as increased transmissibility, decreased neutralization by vaccine promoted antibodies, or possible interference with diagnostic biomarkers.[69] For IVD purposes, variants of concern and interest possess (or are suspect to possess) mutations that lie in a portion of the viral genome that is known to produce proteins directly detected by antigen tests, or indirectly via antibodies produced in response to viral proteins. Mutants possess new amino acid sequences that may cause a shift in the conformation of the protein, which may hinder the binding of important detection Abs. If detection Abs do not bind their target antigen by the time they reach the capture Abs at the test line of a LFA, the AuNP conjugate will fail to be immobilized to the nitrocellulose. Instead of a solid red line to denote an infected individual, the AuNPs will flow past into the absorbent pad leaving the person to believe the test is negative.
A mutagen named B.1.1.7 (Alpha) variant, grew to be the most dominant form in the United States by Spring 2021 having 50% increased transmissibility from the original 2019 strain owing to 12 distinct mutations in the S protein coding region.[69,70] Despite the mutations taking place in a critical biomarker for many LFAs, the largest producers and processers of rapids tests: Abbott, LabCorp, and Quest Diagnostics report no change in the accuracy of diagnostics towards this variant as well as the African and Brazilian variants.[71] A variant under investigation discovered in a small population in French Britanny has been found to be able to evade several diagnostic platforms including the “gold standard” PCR testing.[72] Currently it is unclear whether this strain will evade rapid tests that look for virus specific antigens or antibodies, however it is the current subject of investigation by test developers examining each variant’s mutations on a case-by-case basis.[73]
By June 2021 a new strain officially named B.1.617.2 (Delta) surged its way to become the dominant strain in the U.S., U.K., India, Russian and over a dozen other countries.[74] The Delta strain is a variant is of concern being a double mutant in the S protein coding region. One such mutation, L452R lies within the RBD and renders it 4 times more infectious than the 2019 original strain.[75] A recent study found that some anti-RBD mAbs used as treatment were found to be ineffective in binding to the mutant S protein of the Delta variant, and antibodies from convalescent sera were fourfold less effective in neutralization.[76] The case is the same for the Lambda variant added as a variant of interest June 2021 due to its novel L452Q mutation in the same region.[77] These variants could significantly impact the performance of LFA tests that look for antibodies produced solely against the spike protein such as the Lyher Novel Coronavirus IgG/IgM Antibody Combo Test Kit.[78] For antigen tests, a similar case is true. If a test looks only for the S protein or S protein RBD it may not detect strains that contain the mutation of concern. Luckily, many tests utilize an Ab array that targets the N, or N and S protein simultaneously to avoid the impact of the highly mutagenic S coding region. Mutations that lie in the N protein coding region exist but occur sparingly, with less than 0.15% global frequency as of March 2021.[79]
Cost factor
Although molecular COVID tests (e.g., PCR) are the gold standard with high specificity, their large-scale adoption is restricted by the requirements of special equipment, skilled staff, lengthy turnaround time, and high cost. One distinct advantage of LFA over other diagnostic technologies for COVID testing is its affordability. The low-cost of LFA is largely due to the economic materials, mass production capabilities, and freedom from operational costs seen with other platforms. Particularly, the materials used in LFA tests (see Sect. “Lateral Flow Assay (LFA) of COVID-19: Materials and Theory” above for details) are stable and low cost.[80] To get a relative idea of the cost of COVID testing, one survey over 2,862 hospitals in the United States, the average cost of a nasal swab COVID-19 PCR test was $161 with low and high average prices of $62 and $302, respectively.[81] AdventHealth Centra Care©, a nationally recognized walk-in clinic, bills $175 for a RT-PCR test, and only $65 for a rapid antigen test at the same location.[82]
In the U.S., the FDA authorized the emergency use of several COVID-19 LFAs. One such LFA, Abbott’s BinaxNOW COVID-19 Ag Card is a rapid antigen test with a turnaround time of 15 min.[83] It is available to consumers at only $5 per test. To put this in perspective, a company of 500 employees testing each employee just once a week would accrue a yearly testing bill upwards of $3.8 million if paying the national average for a nasal swab PCR test, compared to ~ $120,000 for Abbott’s COVID-19 Ag Card. Another LFA is the q-SARS-CoV-2 IgG/IgM antibody test manufactured by Cellex. Each test kit comes with materials to run 25 tests with each test costing $20 and the result can be obtained in 15–20 min.[84] In December 2020, the U.S. FDA issued EUA for the first over-the-counter fully at-home diagnostic test for COVID-19 – the Ellume COVID-19 Home Test.[85] This is an LFA antigen test with a price tag of $45 and a turnaround time of 15 min.[86] A study commissioned by the America’s Health Insurance Plans (AHIP) estimated that the annual COVID-19 testing costs in U.S. could reach $25 billion if molecular or antigen tests are used while the number drops to $19 billion if antibody tests are used. Widely adopting LFA tests can lead to significant testing cost savings in the U.S. and around the world.[87]
Societal impact
LFA technology is vital to the efforts to control and manage the ongoing COVID-19 pandemic, primarily because it is user-friendly and low-cost. The LFA technology can be particularly useful in diagnosing COVID-19 in economically disadvantaged and resource constrained areas, such as developing countries and rural communities. For instance, in areas that lack sufficient expensive and sophisticated PCR equipment and/or trained technicians who can perform the PCR test, the use of LFA technology not only can help greatly expand access to testing, but also significantly reduce the economic burden associated with mass testing. Additionally, LFA tests can return results within minutes, much faster than those of PCR tests. Given the highly infectious nature of the COVID-19 disease, increased capacity of testing that requires less reaction time, such as LFA tests, can help speed up the tracing, triaging and treating of patients, and thus is essential in the monitoring and control of COVID-19 worldwide and particularly, in the under-resourced areas.[88]
Moreover, LFA tests may serve as a valuable preventative testing tool in the severely impacted long-term care facilities (e.g., nursing homes and assisted living facilities) and custodial settings (e.g., prisons, jails, and immigration detention centers). These facilities, congregate in nature and highly occupied, are susceptible to COVID-19 infection and spread. In addition, a large proportion of individuals in these facilities are at greater risk for serious COVID-19 outcomes. Nursing home residents, for example, are especially vulnerable to severe illness and mortality if infected by COVID-19 due to a host of risk factors, such as being older and having chronic underlying health conditions. As of June 30, 2021, over 185,000 lives of the residents and staff of nursing homes or other long-term care facilities in the U.S. have been taken by COVID-19, accounting for approximately 30% of all COVID-19 deaths in the nation.[89] These numbers highlight the importance of surveillance, prevention and disease control in nursing homes and other congregate settings. Routine testing of staffs, residents, and visitors can contribute to preventing introduction and reducing transmission of COVID-19 in these facilities. In the U.S., nursing facilities have been required by the Centers for Medicare and Medicaid Services (CMS) to perform testing of all staffs on a regular basis (testing intervals ranging from monthly to twice per week depending on local infection rate) between August 2020 and April 2021, and of all unvaccinated staffs since April 2021.[90]
Despite of the relatively lower sensitivity compared to PCR tests, LFA tests are suitable for regular and frequent use as a preventative measure due to their cost-effective, speedy, and user-friendly features. One recent study found that the frequency of employee testing, supplemented with other infection control measures, plays a key role in reducing the number of cases and deaths in long-term care facilities.[91] The findings suggested that frequent testing of employees (i.e., daily) using good quality antigen rapid tests is more effective than less frequent testing (i.e., once every 5 days) using good quality PCR tests. Similarly, a decision analytical modeling study based on simulated nursing home residents and staffs found that antigen tests performed better than PCR tests in reducing transmission at the same testing frequency due to rapid turn-around, and that daily antigen rapid testing was the most effective testing strategy in the reduction in infections.[92] Altogether, these findings indicate that rapid testing could be utilized routinely and frequently as an effective preventative tool to detect infection and reduce transmission of COVID-19 in at-risk congregate facilities.
Challenges and outlook
Ease of use, low-cost, rapid readout time, and mass production capabilities are some of the distinguishing features of LFA rapid tests critical for the containment of the current pandemic. Despite their critical role, LFAs are hampered by weak sensitivity and the possibility of missing infected individuals that could transmit the virus.
The need for highly sensitive detection is particularly evident in COVID-19 as each infected person is estimated to infect multiple individuals.[93] It is speculated with a generational interval and incubation period of ~ 5–13, and ~ 4–5 days, respectively, that most transmissions of COVID-19 occur on or just prior to symptom onset.[93,94] This has been validated in viral shedding studies that peak transmissibility could continue for ~ 10 days, around 7 times longer than seasonal influenza.[95] Therefore, it is critical to improve the sensitivity of LFAs to catch potentially positive individuals before, or without symptom onset. LFA platforms currently approved for emergency use by the FDA have widely variable sensitivities (rate of true positives). The FDA estimates of serological LFA performance vary in a wide range between 90.0 and 100% for sensitivity (see Table 1, for examples).[96] Antigen tests also have been known to have issues with high rates of false positives as addressed by the FDA.[97] Data presented by the FDA claims sensitivities of 90% or higher however, this is not to be represented as the real-world performance. Independent studies have shown many LFAs’ sensitivities to be lower than originally claimed.[98,99] Deviation of the sensitivity from pre-clinical data could be attributed to factors such as non-random sampling, user error, and local prevalence of disease.[100,101]
It is clear that improving the sensitivity of LFA would greatly benefit public health during the current and future pandemics. Above all other components of LFA technology, the intensity of the signal generating species (i.e., labels) is believed to have a direct, significant impact on the sensitivity of the assay.[102] In principle, if all other variables are kept constant, a more intense signal generating label will provide a heightened sensitivity. To date, the most used COVID-19 LFAs’ signal readout depends on plasmonic AuNPs as labels to generate a red color. If there is not a significant amount of AuNPs immobilized at the test line (especially when detecting samples of low analyte concentrations), not enough color will be present at the test line to be visually discernable as a positive result. Improvement of the signal generated by AuNP can be achieved by various methods including using AuNP aggregates or AuNPs-decorated carriers as labels that can generate more intense overall color signal compared to individual AuNPs.[103,104] Newly engineered nanomaterials which possess stronger plasmonic activities (such as gold nanoshells[105] and gold-silver hollow nanoparticles[106] might be strong candidates for improving the sensitivity of LFA for COVID-19. Recently, it has been demonstrated that sensitivity of LFA can be substantially enhanced by replacing AuNPs with catalytic nanoparticles, which generate intense color signal by catalyzing chromogenic substrates.[29,107] This new strategy may be applicable to LFA of COVID-19. Notably, those non-colorimetric labels (e.g., fluorescent or magnetic nanoparticles[108,109] are also expected to be able to enhance the sensitivity of COVID LFA. However, additional devices may be needed, such as a fluorescence or magnetic field detector, which compromises the convenience of LFA technology.
It is worth emphasizing that the possibility of new coronavirus mutations interfering with LFA diagnostics is always looming. In February 2021 the FDA established guidance on the evaluation of diagnostics tests towards new mutant COVID-19 strains.[110] This guidance calls for companies holding EUA to evaluate IVD platforms for effectiveness towards variants and to develop tests around them. Most LFA rapid tests will still have efficacy towards a majority of the coronavirus infected population, especially in tests that look for multiple biomarkers or biomarkers other than the S Protein, where mutations are most common. Nevertheless, it is critical to investigate new variants, and develop effective testing platforms that keep up with the virus’ natural evolutionary process.
To conclude and prospect, during this time of public health crisis due to COVID-19, lateral flow technology finds necessary use to diagnose potentially millions of new cases each year. The cost-effective, lightweight design allows for facile mass production and shipping capabilities, unlike other more equipment heavy diagnostic platforms (e.g., PCR and ELISA). Even though sensitivities of COVID-19 LFAs are not up to par with these tests yet, the highly versatile, engineerable design of LFAs present a special challenge and opportunity to the research community to improve upon them. The ability to study mutant strains’ antibody response in humans lets test designers stay one step ahead of the highly mutagenic virus by simply switching the antibody array to capture the most highly conserved antigens. The future health of the world depends highly on the advancement of rapid COVID-19 diagnostics. Simply put, rapid COVID LFAs have saved countless lives from the battle with COVID-19 and will continue to do so, pending we humans take the effort through research and development to fight back.
References
J. Zheng, SARS-CoV-2: an emerging coronavirus that causes a global threat. Int. J. Biol. Sci. 16(10), 1678 (2020)
Johns Hopkins University and Medical, Coronavirus Resource Center Map. https://coronavirus.jhu.edu/map.html . Accessed 12 May 2021
UNAIDS, Global HIV & AIDS statistics—Fact sheet. https://www.unaids.org/en/resources/fact-sheet . Accessed 12 May 2021
FDA, COVID-19 Tests and Collection Kits Authorized by the FDA in 2020: Infographic (2020). https://www.fda.gov/medical-devices/coronavirus-covid-19-and-medical-devices/covid-19-tests-and-collection-kits-authorized-fda-infographic. Accessed 12 May 2021
H. Ritchie, E. Ortiz-Ospina, D. Beltekian, E. Mathieu, J. Hasell, B. Macdonald, C. Giattino, C. Appel, L. Rodés-Guirao, M. Roser, Coronavirus Pandemic (COVID-19). https://ourworldindata.org/coronavirus. Accessed 3 May 2021
A. Crozier, S. Rajan, I. Buchan, M. McKee, Put to the test: use of rapid testing technologies for covid-19. BMJ (2021). https://doi.org/10.1136/bmj.n208
G.A. Posthuma-Trumpie, J. Korf, A.V. Amerongen, Lateral flow (immuno) assay: its strengths, weaknesses, opportunities and threats. Anal. Bioanal. Chem. 393(2), 569–582 (2009)
H. Ye, X. Xia, Enhancing the sensitivity of colorimetric lateral flow assay (CLFA) through signal amplification techniques. J. Mater. Chem. B 6(44), 7102–7111 (2018)
FDA, In Vitro Diagnostics EUAs (24 May 2021). https://www.fda.gov/medical-devices/coronavirus-disease-2019-covid-19-emergency-use-authorizations-medical-devices/in-vitro-diagnostics-euas . Accessed 03 May 2021
B. O’Farrell, Evolution in lateral flow–based immunoassay systems, in Lateral Flow Immunoassay. (Springer, New York, 2009), pp. 1–33
K.M. Koczula, A. Gallotta, Lateral flow assays. Essays Biochem. 60(1), 111–120 (2016). https://doi.org/10.1042/EBC20150012
D.R. Davies, G.H. Cohen, Interactions of protein antigens with antibodies. Proc. Natl. Acad. Sci. 93(1), 7–12 (1996). https://doi.org/10.1073/pnas.93.1.7
A.F. Ogata, A.M. MaleY, C. Wu, T. Gilboa, M. Norman, R. Lazarovits, C.-P. Mao, G. Newton, M. Chang, K. Nguyen, Ultra-sensitive serial profiling of SARS-CoV-2 antigens and antibodies in plasma to understand disease progression in COVID-19 patients with severe disease. Clin. Chem. 66(12), 1562–1572 (2020)
J.S. Ponti, Material platform for the assembly of lateral flow immunoassay test strips, in Lateral Flow Immunoassay. (Springer, New York, 2009), pp. 1–7
C. Parolo, A. Sena-Torralba, J.F. Bergua, E. Calucho, C. Fuentes-Chust, L. Hu, L. Rivas, R. Álvarez-Diduk, E.P. Nguyen, S. Cinti, Tutorial: design and fabrication of nanoparticle-based lateral-flow immunoassays. Nat. Protoc. 15(12), 3788–3816 (2020)
R. Gerbers, Development of enhanced lateral flow test devices for point-of-care diagnostics. University of Rhode Island: (2014) https://www.proquest.com/docview/1432906219?pq-origsite=gscholar&fromopenview=true . Accessed 9 Aug 2021
N. Iftikhar, What’s a normal blood pH and what makes it change?: (2019) https://www.healthline.com/health/ph-of-blood . Accessed 9 Aug 2021
N. Washington, R. Steele, S. Jackson, D. Bush, J. Mason, D. Gill, K. Pitt, D. Rawlins, Determination of baseline human nasal pH and the effect of intranasally administered buffers. Int. J. Pharm. 198(2), 139–146 (2000)
R. Reverberi, L. Reverberi, Factors affecting the antigen-antibody reaction. Blood Transfus. 5(4), 227 (2007). https://doi.org/10.2450/2007.0047-07
K.H. Ching, Lateral flow immunoassay. ELISA. Methods Mol. Biol. 1318, 127–137 (2015). https://doi.org/10.1007/978-1-4939-2742-5_13
M. Sajid, A.-N. Kawde, M. Daud, Designs, formats and applications of lateral flow assay: a literature review. J. Saudi Chem. Soc. 19(6), 689–705 (2015)
M.A. Mansfield, Nitrocellulose membranes for lateral flow immunoassays: a technical treatise, in Lateral Flow Immunoassay. (Springer, New York, 2009), pp. 1–19
L. Rivas, M. Medina-Sánchez, A. De La Escosura-Muñiz, A. Merkoçi, Improving sensitivity of gold nanoparticle-based lateral flow assays by using wax-printed pillars as delay barriers of microfluidics. Lab. Chip 14(22), 4406–4414 (2014)
T. Přistoupil, M. Kramlova, J. Štěrbíková, On the mechanism of adsorption of proteins to nitrocellulose in membrane chromatography. J. Chromatogr. A 42, 367 (1969)
E. Millipore, Rapid Lateral Flow Test Strips: Considerations for Product Development (EMD Millipore Corporation, Billerica, 2013)
R. Wong, H. Tse, Lateral Flow Immunoassay (Springer Science & Business Media, New York, 2008), p. 103
V. Amendola, R. Pilot, M. Frasconi, O.M. Marago, M.A. Iatì, Surface plasmon resonance in gold nanoparticles: a review. J. Phys. Condens. Matter 29, 203002 (2017)
E.C. Dreaden, A.M. Alkilany, X. Huang, C.J. Murphy, M.A. El-Sayed, The golden age: gold nanoparticles for biomedicine. Chem. Soc. Rev. 41, 2740 (2012)
Z. Gao, H. Ye, D. Tang, J. Tao, S. Habibi, A. Minerick, D. Tang, X. Xia, Platinum-decorated gold nanoparticles with dual functionalities for ultrasensitive colorimetric in vitro diagnostics. Nano Lett. 17, 5572 (2017). https://doi.org/10.1021/acs.nanolett.7b02385
X. Qian, X.-H. Peng, D.O. Ansari, Q. Yin-Goen, G.Z. Chen, D.M. Shin, L. Yang, A.N. Young, M.D. Wang, S. Nie, In vivo tumor targeting and spectroscopic detection with surface-enhanced Raman nanoparticle tags. Nat. Biotechnol. 26, 83 (2008). https://doi.org/10.1038/nbt1377
X. Xia, J. Zhang, N. Lu, M.J. Kim, K. Ghale, Y. Xu, E. McKenzie, J. Liu, H. Ye, Pd–Ir core–shell nanocubes: a type of highly efficient and versatile peroxidase mimic. ACS Nano 9, 9994 (2015)
A. Marques, P. Costa, S. Velho, M. Amaral, Functionalizing nanoparticles with cancer-targeting antibodies: a comparison of strategies. J. Controll Release 320, 180 (2020). https://doi.org/10.1016/j.jconrel.2020.01.035
S. Kumar, J. Aaron, K. Sokolov, Directional conjugation of antibodies to nanoparticles for synthesis of multiplexed optical contrast agents with both delivery and targeting moieties. Nat. Protoc. 3, 314 (2008)
M.H. Jazayeri, H. Amani, A.A. Pourfatollah, H. Pazoki-Toroudi, B. Sedighimoghaddam, Various methods of gold nanoparticles (GNPs) conjugation to antibodies. Sens. Bio-Sens. Res. 9, 17 (2016)
M.C. Brown, Antibodies: key to a robust lateral flow immunoassay, in Lateral Flow Immunoassay. (Springer, New York, 2009), p. 1
C. Zhang, Hybridoma Technology for the Generation of Monoclonal Antibodies. Antibody Methods and Protocols (Springer, New York, 2012), p. 117
N.S. Lipman, L.R. Jackson, L.J. Trudel, F. Weis-Garcia, Monoclonal versus polyclonal antibodies: distinguishing characteristics, applications, and information resources. ILAR J. 46, 258 (2005)
H.F. Stills, Polyclonal antibody production, in The Laboratory Rabbit, Guinea pig, Hamster, and Other Rodents. (Elsevier, Amsterdam, 2012), p. 259
FDA, GenBody COVID-19 Ag Test Instructions for Use (IFU) (2021). https://www.fda.gov/media/150788/download. Accessed 6 Aug 2021
FDA, Assure COVID-19 IgG/IgM Rapid Test Device Instructions for Use (IFU) (2020). https://www.fda.gov/media/139792/download . Accessed 08 Aug 2021
FDA, Cellex qSARS-CoV-2 IgG/IgM rapid test emergency use authorization (EUA) (2020). https://www.fda.gov/media/136625/download . Accessed 5 May 2021
FDA, Healgen COVID-19 IgG/IgM Rapid Test Cassette Emergency Use Authorization (EUA) (2020). https://www.fda.gov/media/138438/download . Accessed 5 May 2021
E.B. Bahadır, M.K. Sezgintürk, Lateral flow assays: principles, designs and labels. TrAC Trends Anal. Chem. 82, 286 (2016). https://doi.org/10.1016/j.trac.2016.06.006
T.C. Tisone, B. O’Farrell, Manufacturing the next generation of highly sensitive and reproducible lateral flow immunoassay, in Lateral Flow Immunoassay. (Springer, New York, 2009), p. 1
N. Dong, X. Yang, L. Ye, K. Chen, E.W.-C. Chan, M. Yang, S. Chen, Genomic and protein structure modelling analysis depicts the origin and infectivity of 2019-nCoV, a new coronavirus which caused a pneumonia outbreak in Wuhan, China. bioRxiv (2020). https://doi.org/10.1101/2020.01.20.913368
B. Alberts, A. Johnson, J. Lewis, M. Raff, K. Roberts, P. Walter, T cells and MHC proteins, Molecular Biology of the Cell. 4th edition Garland Science2002. https://www.ncbi.nlm.nih.gov/books/NBK26926/ . Accessed 13 May 2021
E.W. Hewitt, The MHC class I antigen presentation pathway: strategies for viral immune evasion. Immunology 110, 163 (2003). https://doi.org/10.1046/j.1365-2567.2003.01738.x
A.K. Azkur, M. Akdis, D. Azkur, M. Sokolowska, W. van de Veen, M.C. Brüggen, L. O’Mahony, Y. Gao, K. Nadeau, C.A. Akdis, Immune response to SARS-CoV-2 and mechanisms of immunopathological changes in COVID-19. Allergy 75, 1564 (2020)
FDA, In Vitro Diagnostics EUAs - Serology and Other Adaptive Immune Response Tests for SARS-CoV-2 (2021). https://www.fda.gov/medical-devices/coronavirus-disease-2019-covid-19-emergency-use-authorizations-medical-devices/in-vitro-diagnostics-euas-serology-and-other-adaptive-immune-response-tests-sars-cov-2 . Accessed 28 Mar 2021
H. Hou, T. Wang, B. Zhang, Y. Luo, L. Mao, F. Wang, S. Wu, Z. Sun, Detection of IgM and IgG antibodies in patients with coronavirus disease 2019. Clin. Transl. Immunol. 9, e1136 (2020). https://doi.org/10.1002/cti2.1136
J. Van Elslande, M. Oyaert, S. Ailliet, M. Van Ranst, N. Lorent, Y.V. Weygaerde, E. André, K. Lagrou, S. Vandendriessche, P. Vermeersch, Longitudinal follow-up of IgG anti-nucleocapsid antibodies in SARS-CoV-2 infected patients up to eight months after infection. J. Clin. Virol. 136, 104765 (2021). https://doi.org/10.1016/j.jcv.2021.104765
C.-K. Chang, M.-H. Hou, C.-F. Chang, C.-D. Hsiao, T.-H. Huang, The SARS coronavirus nucleocapsid protein–forms and functions. Antivir. Res. 103, 39 (2014)
Z. Liu, X. Xiao, X. Wei, J. Li, J. Yang, H. Tan, J. Zhu, Q. Zhang, J. Wu, L. Liu, Composition and divergence of coronavirus spike proteins and host ACE2 receptors predict potential intermediate hosts of SARS-CoV-2. J. Med. Virol. 92, 595 (2020)
D. Shan, J.M. Johnson, S.C. Fernandes, H. Suib, S. Hwang, D. Wuelfing, M. Mendes, M. Holdridge, E.M. Burke, K. Beauregard, N-protein presents early in blood, dried blood and saliva during asymptomatic and symptomatic SARS-CoV-2 infection. Nat. Commun. 12(1), 1931 (2021)
FDA, FDA briefing document: Moderna COVID-19 vaccine (17 December 2020). Vaccines and Related Biological Products Advisory Committee Meeting. https://www.fda.gov/media/144434/download . Accessed 08 Aug 2021
FDA, FDA Briefing Document: Pfizer-BioNTech COVID-19 Vaccine (10 December 2020). Vaccines and Related Biological Products Advisory Committee Meeting. https://www.fda.gov/media/144245/download . Accessed 08 Aug 2021
N. Kitchin, Pfizer/BioNTech COVID-19 mRNA vaccine (2020). https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2020-08/Pfizer-COVID-19-vaccine-ACIP-presentation-508.pdf . Accessed 10 Aug 2021
B. Zhang, D. Yue, Y. Wang, F. Wang, S. Wu, H. Hou, The dynamics of immune response in COVID-19 patients with different illness severity. J. Med. Virol. 93, 1070 (2021). https://doi.org/10.1002/jmv.26504
S. Sami, L.J. Akinbami, L.R. Petersen, A. Crawley, S.L. Lukacs, D. Weiss, R.A. Henseler, N. Vuong, L. Mackey, A. Patel, Prevalence of SARS-CoV-2 antibodies in first responders and public safety personnel, New York City, New York, USA, May–July 2020. Emerg. Infect. Dis. 27, 796 (2021)
T. Bradley, E. Grundberg, R. Selvarangan, C. LeMaster, E. Fraley, D. Banerjee, B. Belden, D. Louiselle, N. Nolte, R. Biswell, Antibody responses after a single dose of SARS-CoV-2 mRNA vaccine. N. Engl. J. Med. 384, 1959 (2021)
NCIRD, Division of viral diseases: myths and facts about COVID-19 vaccines (15 April 2021). https://www.cdc.gov/coronavirus/2019-ncov/vaccines/facts.html . Accessed 05 May 2021
S.E. Oliver, J.W. Gargano, H. Scobie, M. Wallace, S.C. Hadler, J. Leung, A.E. Blain, N. McClung, D. Campos-Outcalt, R.L. Morgan, The advisory committee on immunization practices’ interim recommendation for use of Janssen COVID-19 vaccine—United States, February 2021. Morb. Mortal. Wkly. Rep. 70, 329 (2021)
Michigan University, What You Need to Know about the Janssen COVID-19 Vaccine (2021). Michigan Medicine. http://www.med.umich.edu/1libr/UMMG/WYNTK_Janssen_COVID_vaccine.pdf . Accessed 5 May 2021
A. F. Ogata, C.-A. Cheng, M. Desjardins, Y. Senussi, A. C. Sherman, M. Powell, L. Novack, S. Von, X. Li, L. R. Baden: Circulating SARS-CoV-2 vaccine antigen detected in the plasma of mRNA-1273 vaccine recipients. Clin Infect Dis: Infectious Diseases Society of America (2021). https://www.embassyphysicians.com/wp-content/uploads/2021/06/Circulating-SARS-CoV-2-Vaccine-Antigen-Detected-in-the-Plasma-of-mRNA-1273-Vaccine-Recipients.pdf
D. Shan, J.M. Johnson, S.C. Fernandes, H. Suib, S. Hwang, D. Wuelfing, M. Mendes, M. Holdridge, E.M. Burke, K. Beauregard, N-protein presents early in blood, dried blood and saliva during asymptomatic and symptomatic SARS-CoV-2 infection. Nat. Commun. 12, 1 (2021)
FDA, ADVAIT RapCov Rapid COVID-19 Test Instructions for Use (2021). https://www.fda.gov/media/145080/download . Accessed 18 June 2021
R.K. Pathan, M. Biswas, M.U. Khandaker, Time series prediction of COVID-19 by mutation rate analysis using recurrent neural network-based LSTM model. Chaos Solitons Fractals 138, 110018 (2020). https://doi.org/10.1016/j.chaos.2020.110018
R. Wang, Y. Hozumi, C. Yin, G.-W. Wei, Mutations on COVID-19 diagnostic targets. Genomics 112, 5204 (2020). https://doi.org/10.1016/j.ygeno.2020.09.028
CDC, Global Variants Report, countries that have reported variants of SARS-CoV-2 (2021). https://covid.cdc.gov/covid-data-tracker/#variant-proportions . Accessed 15 June 2021
CDC, SARS-CoV-2 Variant Classifications and Definitions (2021). https://www.cdc.gov/coronavirus/2019-ncov/variants/variant-info.html . Accessed 28 Apr 2021
M. Vann, COVID-19 tests can spot variants, lab companies insist (9 February 2021). http://abcnewsradioonline.com/health-news/covid-19-tests-can-spot-variants-lab-companies-insist.html
S. Robertson, New SARS-CoV-2 variant in France appears undetectable by PCR (17 March 2021). https://www.news-medical.net/news/20210317/New-SARS-CoV-2-variant-in-France-appears-undetectable-by-PCR.aspx . Accessed 15 June 2021
Abbott Newsroom, Evaluating Delta and other COVID variants to ensure test effectiveness (10 August 2021). https://www.abbott.com/corpnewsroom/diagnostics-testing/monitoring-covid-variants-to-ensure-test-effectiveness.html . Accessed 10 Aug 2021
S. Mishra, Why is Delta more infectious and deadly? New research holds answers (2021). https://www.nationalgeographic.com/science/article/why-is-delta-more-infectious-and-deadly-new-research-holds-answers . Accessed 5 Aug 2021
O. Mor, M. Mandelboim, S. Fleishon, E. Bucris, D. Bar-Ilan, M. Linial, Y. Lustig, E. Mendelson, N.S. Zuckerman, The rise and fall of an emerging SARS-CoV-2 variant with the spike protein mutation L452R. medRxiv (2021). https://doi.org/10.1101/2021.07.03.21259957
D. Planas, D. Veyer, A. Baidaliuk, I. Staropoli, F. Guivel-Benhassine, M.M. Rajah, C. Planchais, F. Porrot, N. Robillard, J. Puech, Reduced sensitivity of SARS-CoV-2 variant delta to antibody neutralization. Nature (2021). https://doi.org/10.1038/s41586-021-03777-9
M.L. Acevedo, L. Alonso-Palomares, A. Bustamante, A. Gaggero, F. Paredes, C.P. Cortés, F. Valiente-Echeverría, R. Soto-Rifo, Infectivity and immune escape of the new SARS-CoV-2 variant of interest Lambda. medRxiv (2021). https://doi.org/10.1101/2021.06.28.21259673
FDA, LYHER Novel Coronavirus (2019-nCoV) IgM/IgG Antibody Combo Test Kit (Colloidal Gold) IFU (2020). https://www.fda.gov/media/139410/download . Accessed 15 July 2021
J. Singh, J. Samal, V. Kumar, J. Sharma, U. Agrawal, N.Z. Ehtesham, D. Sundar, S.A. Rahman, S. Hira, S.E. Hasnain, Structure-function analyses of new SARS-CoV-2 variants B. 1.1.7, B. 1.351 and B. 1.1. 28.1: clinical, diagnostic, therapeutic and public health implications. Viruses 13, 439 (2021). https://doi.org/10.3390/v13030439
B.G. Andryukov, Six decades of lateral flow immunoassay: from determining metabolic markers to diagnosing COVID-19. AIMS Microbiol. 6, 280 (2020). https://doi.org/10.3934/microbiol.2020018
A. Pavoola, The average cost of a hospital COVID-19 test in each state (2020). https://www.beckershospitalreview.com/finance/the-average-cost-of-a-hospital-covid-19-test-in-each-state.html . Accessed 19 July 2021
Abbott Newsroom, Abbott's fast, $5, 15-minute, easy-to-use COVID-19 Antigen Test Receives FDA Emergency Use Authorization (2020). https://abbott.mediaroom.com/2020-08-26-Abbotts-Fast-5-15-Minute-Easy-to-Use-COVID-19-Antigen-Test-Receives-FDA-Emergency-Use-Authorization-Mobile-App-Displays-Test-Results-to-Help-Our-Return-to-Daily-Life-Ramping-Production-to-50-Million-Tests-a-Month?cid=DISP_BN_US_TW_13 . Accessed 08 Aug 2021
COVID-19 Testing at Orlando International Airport (2021). https://centracare.adventhealth.com/urgent-care/covid-19-testing-orlando-international-airport . Accessed 13 Aug 2021
Cellex, CELLEX QSARS-COV-2 IgG/IgM RAPID TEST KIT (2020). https://cellex.com/product/cellex-qsars-cov-2-iggigm-rapid-test-kit/2/ . Accessed 08 Aug 2021
FDA, Coronavirus (COVID-19) update: FDA authorizes antigen test as first over-the-counter fully at-home diagnostic test for COVID-19 (2020). https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-antigen-test-first-over-counter-fully-home-diagnostic . Accessed 10 Aug 2021
Everlywell, Ellume COVID-19 home test. https://www.everlywell.com/products/covid-ellume-rapid-test/ . Accessed 10 Aug 2021
K. Grow, New study: COVID-19 testing costs could reach $25 billion a year for diagnostic, $19 billion a year for antibody (2020). AHIP. https://www.ahip.org/new-study-covid-19-testing-costs/ . Accessed 10 Aug 2021
A. Olalekan, B. Iwalokun, O.M. Akinloye, O. Popoola, T.A. Samuel, O. Akinloye, COVID-19 rapid diagnostic test could contain transmission in low-and middle-income countries. Afr. J. Lab. Med. 9, 1 (2020). https://doi.org/10.4102/ajlm.v9i1.1255
KFF, State COVID-19 Data and Policy Actions (2021). https://www.kff.org/coronavirus-covid-19/issue-brief/state-covid-19-data-and-policy-actions/. Accessed 10 Aug 2021
CMS, Interim Final Rule (IFC), CMS-3401-IFC, Additional Policy and Regulatory Revisions in Response to the COVID-19 Public Health Emergency related to Long-Term Care (LTC) Facility Testing Requirements and Revised COVID19 Focused Survey Tool (2020). https://www.cms.gov/files/document/qso-20-38-nh.pdf . Accessed 23 July 2021
H.C.J. Tsoungui Obama, N. Adil Mahmoud Yousif, L. Alawam Nemer, P.M. Ngougoue Ngougoue, G.A. Ngwa, M. Teboh Ewungkem, K.A. Schneider, Preventing COVID-19 spread in closed facilities by regular testing of employees: an efficient intervention in long-term care facilities and prisons? PLoS ONE 16, e0249588 (2021). https://doi.org/10.1371/journal.pone.0249588
I. Holmdahl, R. Kahn, J.A. Hay, C.O. Buckee, M.J. Mina, Estimation of transmission of COVID-19 in simulated nursing homes with frequent testing and immunity-based staffing. JAMA Netw. Open 4, e2110071 (2021). https://doi.org/10.1001/jamanetworkopen.2021.10071
Q. Li, X. Guan, P. Wu, X. Wang, L. Zhou, Y. Tong, R. Ren, K.S. Leung, E.H. Lau, J.Y. Wong, Early transmission dynamics in Wuhan, China, of novel coronavirus–infected pneumonia. N. Engl. J. Med. (2020). https://doi.org/10.1056/NEJMoa2001316
T. Ganyani, C. Kremer, D. Chen, A. Torneri, C. Faes, J. Wallinga, N. Hens, Estimating the generation interval for coronavirus disease (COVID-19) based on symptom onset data. Eurosurveillance 25, 2000257 (2020). https://doi.org/10.2807/1560-7917.ES.2020.25.17.2000257
X. He, E.H. Lau, P. Wu, X. Deng, J. Wang, X. Hao, Y.C. Lau, J.Y. Wong, Y. Guan, X. Tan, Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat. Med. 26, 672 (2020). https://doi.org/10.1038/s41591-020-0869-5
FDA, EUA Authorized Serology Test Performance (2021). https://www.fda.gov/medical-devices/coronavirus-disease-2019-covid-19-emergency-use-authorizations-medical-devices/eua-authorized-serology-test-performance . Accessed 23 July 2021
FDA, Potential for false positive results with antigen tests for rapid detection of SARS-CoV-2 - Letter to Clinical Laboratory Staff and Health Care Providers (2020). https://www.fda.gov/medical-devices/letters-health-care-providers/potential-false-positive-results-antigen-tests-rapid-detection-sars-cov-2-letter-clinical-laboratory . Accessed 24 July 2021
Wise, Jaqui, Covid-19: Lateral flow tests miss over half of cases, Liverpool pilot data show (2020). https://www.bmj.com/content/371/bmj.m4848 . Accessed 28 Apr 2021
Flower, Barnaby, Clinical and laboratory evaluation of SARS-CoV-2 lateral flow assays for use in a national COVID-19 seroprevalence survey (2020). https://thorax.bmj.com/content/75/12/1082 . Accessed 28 Apr 2021
H. Brenner, O. Gefeller, Variation of sensitivity, specificity, likelihood ratios and predictive values with disease prevalence. Stat. Med. 16, 981 (1997). https://doi.org/10.1002/(SICI)1097-0258(19970515)16:9%3c981::AID-SIM510%3e3.0.CO;2-N
Z.C. Brooks, S. Das, COVID-19 testing: impact of prevalence, sensitivity, and specificity on patient risk and cost. Am. J. Clin. Pathol. 154, 575 (2020). https://doi.org/10.1093/ajcp/aqaa141
H. Ye, X. Xia, Enhancing the sensitivity of colorimetric lateral flow assay (CLFA) through signal amplification techniques. J. Mater. Chem. B 6, 7102 (2018). https://doi.org/10.1039/C8TB01603H
J. Hu, L. Wang, F. Li, Y.L. Han, M. Lin, T.J. Lu, F. Xu, Oligonucleotide-linked gold nanoparticle aggregates for enhanced sensitivity in lateral flow assays. Lab. Chip 13, 4352 (2013). https://doi.org/10.1039/C3LC50672J
H. Xu, J. Chen, J. Birrenkott, J.X. Zhao, S. Takalkar, K. Baryeh, G. Liu, Gold-nanoparticle-decorated silica nanorods for sensitive visual detection of proteins. Anal. Chem. 86(15), 7351–7359 (2014)
S. Jeong, M.W. Kim, Y.R. Jo, N.Y. Kim, D. Kang, S.Y. Lee, S.Y. Yim, B.J. Kim, J.H. Kim, Hollow porous gold nanoshells with controlled nanojunctions for highly tunable plasmon resonances and intense field enhancements for surface-enhanced Raman scattering. ACS Appl. Mater. Interfaces 11(47), 44458–44465 (2019)
Z. Gao, H. Ye, Q. Wang, M.J. Kim, D. Tang, Z. Xi, Z. Wei, S. Shao, X. Xia, Template regeneration in galvanic replacement: a route to highly diverse hollow nanostructures. ACS Nano 14, 791 (2020). https://doi.org/10.1021/acsnano.9b07781
C.N. Loynachan, M.R. Thomas, E.R. Gray, D.A. Richards, J. Kim, B.S. Miller, J.C. Brookes, S. Agarwal, V. Chudasama, R.A. McKendry, M.M. Stevens, Platinum nanocatalyst amplification: redefining the gold standard for lateral flow immunoassays with ultrabroad dynamic range. ACS Nano 12, 279 (2018). https://doi.org/10.1021/acsnano.7b06229
Z. Li, Y. Wang, J. Wang, Z. Tang, J.G. Pounds, Y. Lin, Rapid and sensitive detection of protein biomarker using a portable fluorescence biosensor based on quantum dots and a lateral flow test strip. Anal. Chem. 82, 7008 (2010). https://doi.org/10.1021/ac101405a
D.B. Wang, B. Tian, Z.P. Zhang, X.Y. Wang, J. Fleming, L.J. Bi, R.F. Yang, X.E. Zhang, Detection of Bacillus anthracis spores by super-paramagnetic lateral-flow immunoassays based on “Road Closure.” Biosens. Bioelectron. 67, 608–614 (2015)
FDA, SARS-CoV-2 viral mutations: impact on COVID-19 Tests (2021). https://www.fda.gov/medical-devices/coronavirus-covid-19-and-medical-devices/sars-cov-2-viral-mutations-impact-covid-19-tests . Accessed 07 July 2021
FDA, In vitro diagnostics EUAs—molecular diagnostic tests for SARS-CoV-2 (2021). https://www.fda.gov/medical-devices/coronavirus-disease-2019-covid-19-emergency-use-authorizations-medical-devices/in-vitro-diagnostics-euas-molecular-diagnostic-tests-sars-cov-2 . Accessed 12 June 2021
FDA, In vitro diagnostics EUAs—antigen diagnostic tests for SARS-CoV-2 (2021). https://www.fda.gov/medical-devices/coronavirus-disease-2019-covid-19-emergency-use-authorizations-medical-devices/in-vitro-diagnostics-euas-antigen-diagnostic-tests-sars-cov-2 . Accessed 12 June 2021
Acknowledgments
This work was supported in part by the 2021 University of Central Florida (UCF) seed funding program.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing financial interest.
Rights and permissions
About this article
Cite this article
Biby, A., Wang, X., Liu, X. et al. Rapid testing for coronavirus disease 2019 (COVID-19). MRS Communications 12, 12–23 (2022). https://doi.org/10.1557/s43579-021-00146-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/s43579-021-00146-5