Abstract
Tricalcium phosphates (TCPs) are of great interest in dental tissue engineering applications. The objective of this study was to incorporate magnesium (Mg) at different concentrations of 0.25, 0.50, 1.00, 2.50, and 5.00 wt.% to TCP and evaluate the effects on phase composition, density, and in vitro interaction with human dental pulp stem cells (hDPSCs). Our results showed that adding Mg stabilized the β-TCP crystal structure and increased the density of the TCP. Mg addition had no adverse effect on hPDSCs response. Although cellular proliferation was slightly less on Mg-TCPs at early time points, it increased significantly with time and in a dose-dependent manner. A similar trend was observed for cellular differentiation. Together, these data show for the first time that Mg addition at concentrations less than 2.50% (preferably at 1.00 or 2.50 wt.%) can be added safely to TCP for enhanced proliferation and differentiation of hDPSCs.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The success of calcium phosphates (CaPs) is due to their high biocompatibility, controllable biodegradability, osteoinductivity potential, which is the ability to guide new bone growth along the material, and their composition similarity to the inorganic portion of the bone, which contributes to approximately 70% of bone’s structure [1, 2]. As a result, CaPs have a wide variety of applications as bone and dental substitutes and are implemented in bone grafts, dental implants, and cements [2]. Using different types of CaPs is directly related to the phase composition, which is defined by its calcium to phosphorous ion ratio and the resulting characteristics like crystallinity, bioresorbability, mechanical properties, and biological responses [1, 2].
Tricalcium phosphate (TCP) has a Ca/P ratio of 1.5 and is more osteoinductive and more soluble than hydroxyapatite [3]. As a result, TCPs tend to be specifically used for biodegradable characteristics to encourage the replacement of the implant with new tissue as it deteriorates [4, 5]. Different methods have been investigated to optimize features like mechanical strength, sintering temperature, density, and cellular interaction with the material in vitro and in vivo. One of the most popular techniques uses a single or a combination of trace elements as dopants such as magnesium, strontium, silicon, lithium, and iron to substitute calcium with foreign ions in the TCP structure; this results in the adaptation of various properties and encourages specific biological responses [3, 6,7,8,9,10]. The current research focuses on modifying the TCP composition by ionic substitution for potential dental applications.
Magnesium is the fourth most abundant ion in the human body, 60% of which can be found in the bone. It is an essential element in bone and dental health. Its concentration is 0.2, 1.1, and 0.6 wt.% in enamel, dentin, and bone, respectively [3]. Magnesium has diverse functions that vary from enzyme activity, support in the bone crystal structure, regulating growth, and intercellular pathway communication [11, 12]. It is also critical in body interaction with vitamin D, parathyroid hormone secretion, and the regulation of osteoclast and osteoblast activity in the absorption and creation of bone [12]. An imbalance due to elevated bone absorption and decreased formation of bone can cause serious health problems due to fragility and an increased risk of injuries such as fractures and possible disability [1, 2, 13]. This correlation has been deduced from studies that have concluded deficiency in magnesium leads to the weakened bone, decreased bone mass and growth, and osteoporosis [14]. Mg is also a pivotal component in dental health and plays a crucial role in tooth apatite formation. Interestingly, the concentration of Mg is different at different parts of the tooth; a higher concentration in dentin (compared to enamel) is associated with dentin’s organic compound [15]. In addition, Mg content on the surface of enamel is less than in the deeper layers, which is due to the mineralization and demineralization process at the surface, as opposed to the deeper layer [16]. Studies have shown that high concentrations of Mg serum by oral intake decreases the possibility of attachment loss and probing health, and improves teeth lifetime, suggesting effective nutritional role of Mg supplement on overall periodontal health [17]. Lack of access to Mg disrupts the intercellular hemostasis and causes significant decrease in proliferation, migration, and osteogenic differentiation of human dental pulp stem cells [18]. The purpose of this research is to investigate the role of Mg addition at different concentrations on physical properties and in vitro interaction with human dental pulp stem cells (hDPSCs).
We hypothesize that the presence of magnesium in TCP will alter its physical properties and in vitro interaction (attachment, proliferation, and differentiation) with hPDSCs. To validate our hypothesis, Mg was added to TCP precursors in several concentrations using the solid-state synthesis method, and TCP disk samples were prepared by uniaxial pressing. The phase composition and density of the samples were analyzed using X-ray diffraction and Archimedes’ methods, respectively. The hDPSCs interaction with scaffolds was examined through SEM, fluorescence microscopy, MTT, and ALP assays. The role of Mg in calcium phosphate ceramics on bone turnover has been studied extensively, and we have already reported the in vitro and in vivo findings [6,7,8, 19,20,21]; however, the presence and optimum concentration of Mg for proliferation and differentiation of hDPSCs are not reported.
Materials and methods
Pure and Mg-doped TCP preparation
Pure and doped TCP powder was fabricated through a solid-state synthesis method using calcium carbonate CaCO3 (≥ 99.0%, Sigma-Aldrich), ammonium phosphate diabasic (NH4)2HPO4 (≥ 98%, Sigma-Aldrich), and magnesium oxide MgO (≥ 99.99%, Sigma-Aldrich) as precursors, as explained in detail previously [17]. Briefly, 3 mol of CaCO3 and 2 mol of (NH4)2HPO4 were milled and mixed at powder:milling media of 4:1 for 2 h. Powders were then calcinated at 1025 °C for 2.5 h, then mixed with ethanol at powder:ethanol:milling media ratio of 1:1.5:5 for 6 h, followed by drying at 70 °C for 72 h. Mg-doped TCP powders with Mg content of 0.25, 0.50, 1.00, 2.50, 5.00, and 7.50 wt.%, were synthesized by the same method, and MgO was added as an Mg precursor while maintaining the (Ca + Mg)/P = 1.5. The dried powder was then pressed to disk samples of approximately 12 mm diameter and 2.5 mm height using a uniaxial press at 25 kN for 2 min, followed by sintering at 1200 °C for 2 h.
Phase analysis and density measurement
The phase composition of a calcined powder and sintered samples was analyzed using a Miniflex Rigaku X-Ray diffraction instrument, with a source of Cu Kα radiation at a step size of 0.05° and a time per step of 1 s. Relative bulk and apparent densities of samples (except for the 7.50 wt.% TCP) were measured by Archimedes’ method. The dry weight and dimension of disk samples were measured at room temperature. Then each disk was submerged individually into boiling deionized water for two minutes, room-temperature water, to measure the wet weight. The average density of at least six samples was calculated as each sample's density.
In vitro human dental pulp stem cells (hDPSCs) culture
Human dental pulp stem cells (hDPSCs) which were previously characterized [22], were transferred to a flask containing DMEM with 4.5 g/L glucose, L-glutamine, and sodium pyruvate (Corning) supplemented with 10% fetal bovine serum (FBS, Gibco Invitrogen) and 1% Antibiotic–antimycotic (HyClone)) and incubated at 37 °C with 5% CO2 and 95% humidity. Cells at 80% confluency were detached using 0.25% trypsin and counted using the C-Chip DHC-NO1 hemocytometer (INCYTO, Korea).
Cells at passage 4, were seeded on the sterilized scaffold at a concentration of 2.5 × 105 cells/samples in 100 µl of culture medium. After one hour of incubation (to allow cell adhesion), samples were immersed in media and incubated at 37 °C and 5% CO2 for predetermined times (1, 3, and 7 days). The culture medium was changed to a fresh medium every other day during the experiment.
Cell attachment, viability, proliferation, and alkaline phosphatase assay
Cell adhesion on the scaffolds was investigated qualitatively by scanning electron microscopy (SEM). After 7 days, samples were removed from culture media, rinsed with PBS, and fixed in the Karnovsky's fixative for 24 h at 4 °C, followed by PBS washing, and dehydration in a series of increasing concentrations of ethanol (30, 50, 70, 90, and 100%v/v). Samples were dried, sputter-coated with gold, and observed under the microscope (SEM, JEOL-JSM6510, Japan).
The viability of DPSCs seeded on scaffolds was assessed using a Live/Dead® viability/cytotoxicity kit (Invitrogen). After 24 h of cell seeding, samples were rinsed with PBS and incubated in a mixture of 5 µM calcein-AM and 4 µM ethidium homodimer-1 (EthD-1) in PBS for 15 min at room temperature. After washing again with PBS, live (green) and dead (red) cells were imaged with fluorescence microscopy (Evos Fluorescent, life technologies).
Presto Blue assay (Invitrogen, Life Technologies) was used as a product directed to measure the proliferation of the samples. After washing scaffolds and cells with PBS and Presto Blue solution (1:9 in DMEM without phenol-red), samples were incubated at 37 °C with 95% humidity and 5% CO2 for 2 h. Once removed, 100 μl of each solution was transferred into a 96-well plate compartment and analyzed by fluorescence intensity at 540 nm and 590 nm with a microplate reader (SYNERGY-HTX, Biotek, USA).
The ALP assay was performed once after the 7th day. Scaffold samples were washed with cold PBS and suspended in ALP assay buffer (Abcam, USA). Cells were lysed three times through freeze–thaw cycles. The cell lysate was then transferred to a new tube and centrifuged at 1000 times gravity for 10 min at 4 °C. The lysates were mixed with pNPP (4-nitrophenyl phosphate) per the manufacturer’s instructions. The absorbance was measured at 405 nm. ALP readings were compared to a standard curve, respectively, and the calculated ALP concentration was normalized to the protein content for each well. The data were plotted after normalizing the nmol pNPP/ug protein.
Statistical analysis
Data for relative bulk density and relative apparent density are presented as mean ± standard deviation. Statistical analysis was performed using Prism 8.0 Software (GraphPad, CA, USA) on PrestoBlue and ALP assay results with a one-way analysis of variance (ANOVA) followed by Tukey's post hoc test'.P values < 0.05 were considered statistically significant.
Results
Phase analysis
Figures 1(a) and (b) show the XRD spectra of pure and Mg-doped TCP at 1025 °C and 1200 °C, respectively. The addition of Mg to TCP and calcination at 1025 °C does not affect the phase composition and all samples contain β-TCP. Sintering the pure TCP at 1200 °C results in the formation of α-TCP as the minor phase, while Mg-doped samples include Mg substituted TCP, magnesium whitlockite ((Ca, Mg)3(PO4)2), as the only phase. As noted in Fig. 1(b), increasing the concentration of Mg slightly shifts the peaks to higher angles.
Density
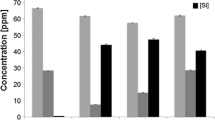
Relative bulk and apparent densities of pure and Mg-substituted TCP are presented in Table 1. The addition of Mg increases the relative bulk density of samples. Incorporation of 0.25 wt.% Mg increases the relative bulk density of samples from 58.7 ± 0.6% to 73.1 ± 0.6%. The highest relative bulk density is measured for 5.00 wt.% Mg-TCP with the value of 80.6 ± 0.9%. Relative apparent density for all samples is higher than the bulk density and in the range of 91–96%.
In vitro hDPSCs attachment, viability, proliferation, and differentiation
To understand the cell-material interaction, the morphology of the cells and their viability were evaluated by SEM and a fluorescence microscope, respectively. Figure 2 shows the morphology of the cells after 24 h of culture. Cells have been attached to all samples regardless of the composition, and the filopodia extensions are visible. Fluorescence microscopy images in Fig. 3 confirm the results by SEM, where several live cells and a few dead/damaged cells were present on all samples. No significant difference was observed in hDPSCs population density between different compositions.
To quantify the cell proliferation, a PrestoBlue assay was used on days 1, 3, and 7 (Fig. 4). On days 1 and 3, the pure sample had the highest cell density. The addition of Mg resulted in a significant reduction of cell proliferation in samples compared to the pure sample except for 1.00 wt.% Mg that didn't show a significant difference with the control group on days 1 and 3 (p > 0.05). While maintaining the same trend, all the Mg-doped samples showed an increase in cell proliferation with time, and the highest cell density was on day 7, which was significantly higher than on day 3. Among all samples, 1.0 Mg-TCP had the highest cell density at all time points as compared to the other Mg-doped samples, although it wasn't significant (p > 0.05). On day 7, 1.0 Mg-TCP showed a significantly higher cell proliferation as compared to the other Mg-doped samples (except for 0.5 Mg and 2.5 Mg) and even in comparison to the control group (p < 0.05).
The differentiation of the hDPSCs in the presence of pure and Mg-doped samples after 7 days of culture was evaluated by ALP assay and the results are presented in Fig. 5. The presence of Mg enhances the ALP activity in a dose-dependent manner. Although the difference between the control group, 0.25 Mg-TCP, and 5.0 Mg-TCP was not statistically significant (p > 0.05), adding 0.5, 1.00, and 2.50 wt.% Mg enhances the ALP expression significantly (p < 0.05). The highest ALP expression was in 1.0 Mg-TCP, which was similar to 0.5 and 2.5 Mg-TCP (p > 0.05). However, a higher concentration of 5.00 wt.% decreases the activity to a similar level to that of the pure sample.
Disscussion
Current results show that the presence of Mg and its concentration alter the phase composition, density, and in vitro interactions with hDPSCs. Adding Mg at the lowest concentration of 0.25 wt.% stabilizes the β-TCP crystal structure and prevents its transformation to α-TCP, which happens at temperatures between 1125 and 1430 °C. Along with the disappearance of α-TCP, a slight shift in the peaks with the addition of Mg proves successful substitution of the divalent ion, Mg, in TCP crystal structure [23]. Mg with the ionic radius of 0.69 Å, is significantly smaller than Ca2+ (ionic radius of 0.99 Å), causes shrinkage in TCP crystal structure and atomic planes, and shifts the XRD peaks to higher angles, according to Bragg’s equation [24]. The magnesium-substituted TCP phase, known as magnesium whitlockite ((Ca, Mg)3(PO4)2), becomes the dominant phase with increased Mg amounts. However, Mg-substitution results in lattice shrinkage up to ~ 14 mol% substitution and further does not change it [25]; this is in line with our current results, where increased Mg concentrations up to 2.50 wt.% result in peak shifts, and no significant change is observed in 5.0 and 7.5 Mg-TCPs. Instead, new peaks at 2theta of ~ 23 and 23.75 may be attributed to the formation of the new phase; stanfieldite (Ca4Mg5(PO4)6); due to saturation of Mg substitution in TCP crystal structure.
The relative bulk density of all Mg-substituted TCP samples is significantly higher than the pure sample, which is attributed to the decrease in unit cell volume. Previous studies showed that adding Mg increases the TCP density after sintering temperatures lower than 1300 °C [6, 14]. Though the bulk density of samples with Mg concentration up to 2.50 wt.% is not significantly different, the addition of 5.00 wt.% Mg increases the density significantly. This can be due to the formation of the stanfieldite with a density of 3.10 g/cm3, which is higher than that of the β-TCP. However, the addition of Mg does not change the density in a regular manner. This non-uniform change in density is attributed to two counteracting phenomena: 1—formation of magnesium-substituted calcium phosphate that increases the density due to a higher molecular weight of Mg than Ca and decrease in structural volume, and 2—liquid phase formation with the addition of Mg that reduces the density of the structure [26]. The exclusion of the 7.5 Mg-TCP from density measurement was due to partial melting of the sintered samples. Addition of Mg around 16 mol.% results in the formation of a liquid phase in the system [24]. In the current work, the appearance of the liquid phase was initiated at a 5.00 wt.% Mg-TCP sample without a noticeable geometrical change, while the addition of 7.50 wt.%Mg showed a significant distortion, which is in line with the Rietveld refinement method [27]. The relative apparent density of all of the samples is significantly higher than the bulk density, which shows the presence of open porosity in the samples. The highest and lowest open porosity in pure and 5.0 Mg-TCP samples, respectively, representing the effective role of Mg in densifying tricalcium phosphate.
Mg-substituted CaPs have been widely used in bone tissue engineering applications. Several studies have shown the effectiveness of Mg on bone cell proliferation, differentiation, maturation, and overall turnover [6, 8, 14]. However, the role of Mg on dental health and dental pulp stem cell evolution is an ongoing investigation. The goal of this research was to find, for the first time, the optimal magnesium concentration to promote proliferation and differentiation of hDPSCs without compromising the physical properties for potential dental tissue engineering applications. The results from the cell viability, cell attachments, and cellular proliferation suggested that all the Mg-TCP compositions were biocompatible with the initial conditions of the scaffold after 24 h. The fluorescence microscopy images demonstrated a dense population of cells with minimal dead or damaged cells, indicating that initial conditions for the in vitro testing were ideal for supporting cells for a longer time frame. SEM images show long extended lamellipodia were attached to the surface, implying strong cell adhesions. Evidence of the cells fully spreading into the substrate suggested that the cells did not have difficulty in integrating with the scaffold samples or with the neighboring cells. Cellular proliferation is the highest on pure TCP at the early time-point of day 1, with no significant change during the 7 days of study. Although Mg presence does not enhance the early cellular proliferation, all Mg-doped samples show increased proliferation of hDPSCs with time, where the cellular activity on day 7 was significantly higher than on days 1 and 3. The proliferation of cells, increases with an increase in Mg concentration up to 1.00 wt.%, then gradually decreases. Differentiation of the hPDSCs was measured by ALP assay. Figure 5 shows the dose dependency of ALP expression with regard to the Mg concentration; low concentrations of 0.25 and 0.50 wt.% increase it, then the maximum activity is in 1.0 wt. % Mg with no significant difference from 0.5 and 2.5 Mg-TCP. However, a higher concentration of 5.00 wt.%Mg decreases the ALP expression significantly to the values similar to that of the pure samples. These results are in line with the available data in the literature. A recent study has shown that culturing human dental pulp stem cells in a medium containing different concentrations of Mg2+ supplement alters cellular proliferation and differentiation significantly and in a dose-dependent manner. While increasing the Mg2+ concentration to 0.5 mM and 1.0 mM enhances cell proliferation, higher concentrations of 4 mM and 8 mM decrease the proliferation drastically. The addition of 2 mM Mg2+ upregulates the alkaline phosphatase activity and dentinogenesis, while 8 mM of Mg2+ decreases the ALP to a lower level than the control specimens without any supplement [28]. The effect of Mg on the proliferation and differentiation of hDPSCs depends on the magnesium precursor. While the previous study used magnesium chloride, another study showed that enhanced proliferation and differentiation happen only in media containing 0.5 mM of magnesium oxide [29]; this indicates that not only the presence and concentration of magnesium but also the composition of precursor significantly alters the cellular behavior.
These preliminary results show the effects of magnesium as a dopant on the physical properties of TCP, along with in vitro human dental pulp stem cell—TCP interaction for potential use in dental tissue engineering applications. Our findings suggest that the addition of either 1.00 wt.% or 2.50 wt.% provides optimal cellular proliferation and differentiation without compromising the physical properties.
Conclusion
In the current research, several concentrations of Mg were added to TCP to investigate its effects on phase composition, density, and in vitro interaction with hDPSCs. Mg addition stabilizes the crystal structure of β-TCP, and increasing the concentration of Mg results in the formation of Mg-substituted TCP with higher density. Liquid phase formation and deformation were observed in samples with 7.50 wt.% Mg. The proliferation of hDPSCs was not affected at early time points; however, an increase in proliferation was observed in all Mg-doped TCP samples with time. The increase in proliferation with the increase in Mg content depends on Mg amount; it upregulates up to 1.00 wt.% Mg, followed by a decrease with the addition of higher amounts. A similar trend was observed for ALP expression. Together, for the first time, these results suggest that Mg addition up to 5.00 wt.% to TCP has no adverse effect on phase composition, density, and human dental pulp stem cell response, and TCP doped with 1.0 or 2.50 wt.% Mg can be considered dental substitute due to enhanced proliferation and differentiation of hDPSCs.
Data availability
All relevant data have been used in this manuscript.
References
W. Habraken, P. Habibovic, M. Epple, M. Bohner, Calcium phosphates in biomedical applications: materials for the future? Mater. Today 19(2), 69–87 (2016)
A. Laskus, J. Kolmas, Ionic substitutions in non-apatitic calcium phosphates. Int J Mol Sci 18(12), E2542 (2017)
E. Boanini, M. Gazzano, A. Bigi, Ionic substitutions in calcium phosphates synthesized at low temperature. Acta Biomater. 6(6), 1882–1894 (2010)
S. Samavedi, A.R. Whittington, A.S. Goldstein, Calcium phosphate ceramics in bone tissue engineering: a review of properties and their influence on cell behavior. Acta Biomater. 9(9), 8037–8045 (2013)
J.S. Al-Sanabani, A.A. Madfa, F.A. Al-Sanabani, Application of calcium phosphate materials in dentistry. Int. J. Biomater. 2013, 1–12 (2013)
S. Bose, S. Vahabzadeh, D. Banerjee, D. Ke, Enhanced bone remodeling on human osteoblast-osteoclast co-culture system using doped hydroxyapatite plasma coatings for musculoskeletal applications. Mater. Today Commun. (2019). https://doi.org/10.1016/j.mtcomm.2019.05.010
M. Roy, A. Bandyopadhyay, S. Bose, Induction plasma sprayed Sr and Mg doped nano hydroxyapatite coatings on Ti for bone implant. J. Biomed. Mater. Res. B Appl. Biomater. 99B(2), 258–265 (2011)
D. Ke, S. Tarafder, S. Vahabzadeh, S. Bose, Effects of MgO, ZnO, SrO, and SiO2 in tricalcium phosphate scaffolds on in vitro gene expression and in vivo osteogenesis. Mater. Sci. Eng., C 96, 10–19 (2019)
S. Vahabzadeh, V.K. Hack, S. Bose, Lithium-doped β-tricalcium phosphate: effects on physical, mechanical and in vitro osteoblast cell-material interactions. J. Biomed. Mater. Res. B Appl. Biomater. 105(2), 391–399 (2017)
S. Vahabzadeh, S. Bose, Effects of iron on physical and mechanical properties, and osteoblast cell interaction in β-tricalcium phosphate. Ann Biomed Eng 45(3), 819–828 (2017)
J.H.F. de Baaij, J.G.J. Hoenderop, R.J.M. Bindels, Magnesium in man: implications for health and disease. Physiol. Rev. 95(1), 1–46 (2015)
E. O’Neill, G. Awale, L. Daneshmandi, O. Umerah, K.W.-H. Lo, The roles of ions on bone regeneration. Drug Discovery Today 23(4), 879–890 (2018)
S. Erem, A. Atfi, M.S. Razzaque, Anabolic effects of vitamin d and magnesium in aging bone. J Steroid Biochem Mol Biol 193, 105400 (2019)
M. Nabiyouni, T. Brückner, H. Zhou, U. Gbureck, S.B. Bhaduri, Magnesium-based bioceramics in orthopedic applications. Acta Biomater. 66, 23–43 (2018)
M. Okazaki, Tooth and Magnesium, in New perspectives in magnesium research: nutrition and health. ed. by Y. Nishizawa, H. Morii, J. Durlach (Springer, London, 2007), pp.359–366
E. Klimuszko, K. Orywal, T. Sierpinska, J. Sidun, M. Golebiewska, Evaluation of calcium and magnesium contents in tooth enamel without any pathological changes. In vitro preliminary study. Odontology 106(4), 369–376 (2018)
P. Meisel, C. Schwahn, J. Luedemann, U. John, H.K. Kroemer, T. Kocher, Magnesium deficiency is associated with periodontal disease. J Dent Res 84(10), 937–941 (2005)
L. Cui, S.M. Xu, D.D. Ma, B.L. Wu, The effect of TRPM7 suppression on the proliferation, migration and osteogenic differentiation of human dental pulp stem cells. Int Endod J 47(6), 583–593 (2014)
X. Li, M. Wang, W. Zhang, Y. Bai, Y. Liu, J. Meng, L. Zhang, A Magnesium-incorporated nanoporous titanium coating for rapid osseointegration. Int. J. Nanomed. 15, 6593–6603 (2020)
Y. Yan, Y. Wei, R. Yang, L. Xia, C. Zhao, B. Gao, X. Zhang, J. Fu, Q. Wang, N. Xu, Enhanced osteogenic differentiation of bone mesenchymal stem cells on magnesium-incorporated titania nanotube arrays. Coll. Surf B Biointerf. 179, 309–316 (2019)
Y. Yajing, D. Qiongqiong, H. Yong, S. Han, X. Pang, Magnesium substituted hydroxyapatite coating on titanium with nanotublar TiO2 intermediate layer via electrochemical deposition. Appl. Surf. Sci. 305, 77–85 (2014)
M. Rasoulianboroujeni, N. Kiaie, F.S. Tabatabaei, A. Yadegari, F. Fahimipour, K. Khoshroo, L. Tayebi, Dual porosity protein-based scaffolds with enhanced cell infiltration and proliferation. Sci Rep 8(1), 14889 (2018)
(15) Synthesis and characterization of tricalcium phosphate with Zn and Mg based dopants | request PDF [Online]. Available: https://www.researchgate.net/publication/5580940_Synthesis_and_Characterization_of_Tricalcium_Phosphate_With_Zn_and_Mg_Based_Dopants. Accessed: 20 Jun 2022
X. Zhang, F. Jiang, T. Groth, K.S. Vecchio, Preparation, characterization and mechanical performance of dense beta-TCP ceramics with/without magnesium substitution. J Mater Sci Mater Med 19(9), 3063–3070 (2008)
K. Yoshida, Effect of substitutional monovalent and divalent metal ions on mechanical properties of Β-tricalcium phosphate. J. Am. Ceram. Soc (2005). https://doi.org/10.1111/j.1551-2916.2005.00399.x
J. Ando, Phase diagrams of Ca 3 (PO 4) 2 -Mg 3 (PO 4) 2 and Ca 3 (PO 4) 2 -CaNaPO 4 systems. BCSJ 31(2), 201–205 (1958)
R. Enderle, F. Götz-Neunhoeffer, M. Göbbels, F.A. Müller, P. Greil, Influence of magnesium doping on the phase transformation temperature of beta-TCP ceramics examined by rietveld refinement. Biomaterials 26(17), 3379–3384 (2005)
R.M. Salem, C. Zhang, L. Chou, Effect of magnesium on dentinogenesis of human dental pulp cells. Int. J. Biomater. 2021, 1–12 (2021)
R.M. Salem, Part I: dentinogenic effect of magnesium oxide on normal human dental pulp cells: an in vitro study. Madridge J. Dent. Oral. Surg. 6(1), 106–115 (2021)
Acknowledgments
The authors acknowledge support from the department of mechanical engineering and the department of biological sciences at Northern Illinois University.
Funding
We would also like to thank the support from National Institute of Dental & Craniofacial Research of the National Institutes of Health under award number R15DE027533, 1 R56 DE029191 -01A1 and 3R15DE027533-01A1W1.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mommer, A., Tabatabaei, F., Tayebi, L. et al. Role of magnesium on phase composition of tricalcium phosphate and its interaction with human dental pulp stem cells. Journal of Materials Research 38, 228–236 (2023). https://doi.org/10.1557/s43578-022-00851-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/s43578-022-00851-4