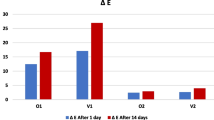
Abstract
Disk-shaped specimens were prepared from additively (NX and DT), subtractively (MZ), and conventionally manufactured denture base resins (CV). Surface roughness and color coordinates were measured after polishing, simulated brushing, and coffee thermocycling, while surface roughness was also measured before polishing. Polishing reduced the surface roughness of all materials. Brushing and coffee thermocycling increased the surface roughness of only DT. CV had the highest susceptibility to consecutive brushing and coffee thermocycling as it had the highest surface roughness, which was above the clinically acceptable threshold. All materials had similar stainability; only MZ had perceptible color change after brushing. Even though stainability of tested denture base resins was similar, additively or subtractively manufactured computer-aided design and computer-aided manufacturing (CAD-CAM) resins had smoother surfaces after brushing and coffee thermocycling, regardless of the material. Therefore, complete dentures made out of these CAD-CAM resins may have favorable surface properties in the long term.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A denture base resin should have optimal flexural strength to prevent fracture and polishability against roughness-related complications such as bacterial adhesion and color instability. Polymethylmethacrylate (PMMA) has been commonly processed by using flask-pack-press manufacturing for the fabrication of removable dentures [1, 2]. Even though conventional PMMA has flexural strength and polishability that can be considered adequate, the fact that it is prone to polymerization-related issues may jeopardize its mechanical properties and lead to bacterial adhesion, discoloration and even cracks and fractures. Parallel with the advancements in computer-aided design and computer-aided manufacturing technologies, indicated deficiencies of conventional PMMA are attempted to be minimized utilizing subtractive manufacturing and prepolymerized pucks for the fabrication of complete dentures [3]. However, despite its advantages, subtractive manufacturing results in excessive waste. More recent additive manufacturing of denture base resins decreased the amount of waste; however, polymerization-related issues may still remain with these types of resins leading to surface-related issues, including roughness and subsequent excessive biofilm formation [4, 5].
Denture cleaning is essential for the longevity of a complete denture [6], regardless of the manufacturing technique being additive, subtractive, or conventional [7], and brushing is a straightforward and affordable cleaning method [8]. However, previous studies have shown the significant effects of brushing on the surface roughness of denture base material [6, 9,10,11,12,13], which is a known cause of plaque accumulation and biofilm formation [14, 15]. Clinically acceptable threshold for surface roughness that would lead to bacterial accumulation has been reported as 0.2 µm in previous in vivo [16] and in vitro studies [3, 5, 6, 9,10,11, 13, 14].
Another critical factor affecting the clinical success of a complete denture is the stainability of the denture base material [17, 18]. Color change may impair the esthetics of a complete denture, which may eventually lead to replacement [19, 20]. Thus, denture base materials should be resistant to exposure to hot and cold beverages not just for stainability but also for the fact that thermal stresses will lead to surface degradation [21]. Even though there are studies on the surface roughness and stainability of additively manufactured denture base resins [2, 7, 22,23,24,25], the number of studies investigating the effect of brushing on surface roughness and stainability is limited [7, 13]. In addition, those studies [7, 13] did not involve a comparison between additively manufactured and subtractively manufactured denture base materials. Therefore, the present study aimed to evaluate the effect of manufacturing technique on the surface roughness and stainability of denture base materials after brushing and coffee thermocycling by comparing additive manufacturing, subtractive manufacturing, and conventional manufacturing. The null hypotheses were that (i) surface roughness of denture base resins would not be affected by the material type (2 additively manufactured, 1 subtractively manufactured, and 1 conventionally manufactured denture base resins) and the time interval (before polishing, after polishing, after brushing, and after coffee thermocycling), and (ii) stainability of denture base resins would not be affected by material type (2 additively manufactured, 1 subtractively manufactured, and 1 conventionally manufactured denture base resins) and time interval (when after polishing stage is compared with after brushing stage and when after polishing stage is compared with after coffee thermocycling stage).
Materials and methods
Specimen preparation
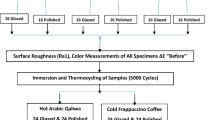
Table 1 lists information and abbreviations regarding the materials used in the present study, while Fig. 1 illustrates the experimental design. A total of 40 disk-shaped specimens (Ø 10 mm × 2 mm) were fabricated by using 4 different denture base resins (NextDent Denture 3D+, NextDent B.V. (NX); Denturetec, Saremco (DT); M-PM Disc, Merz Dental GmbH (MZ); Meliodent Heat Cure, Kulzer GmbH (CV)) (n = 10). The number of specimens in each group was determined based on the results of similar previous studies that reported significant differences [3, 7, 13].
A 10-mm-wide cylinder was designed in standard tessellation language format by using a design software (Meshmixer v3.5.474; Autodesk Inc) for the fabrication of MZ specimens. This design file was used to mill prepolymerized PMMA disks with a milling unit (Milling unit M1; Zirkonzahn). Cylinder-shaped specimens were further wet-sliced by using a precision cutter (Vari/cut VC-50; Leco Corporation) to obtain 2-mm-thick specimens. Same software was used to design a disk-shaped standard tessellation language file with the desired final dimensions to fabricate NX and DT specimens. This standard tessellation language file was transferred into nesting software (RayWare; SprintRay Inc for NX and Composer v1.3.3; Asiga for DT) and positioned with 45° angle to the build platform. After automatically generating supports, this configuration was duplicated 10 times and the specimens were printed by using digital light processing printers (MoonRay S100; SprintRay Inc for NX and MAX UV; Asiga for DT). All specimens were printed with a layer thickness of 50 µm. All 3D-printing processes were performed in a specifically designed room that had stabilized temperature (20 °C) and humidity (45%), which were controlled by building maintenance system. Patterns with the desired final dimensions were prepared from wax and processed according to the traditional flask-press-pack technique (heat polymerization at 74 °C for 8 h) for the fabrication of CV specimens [2]. After deflasking, excess material was trimmed and all specimens were smoothened by using #600 silicon carbide abrasive papers under running water. A uniform final thickness (2 ± 0.03 mm) was ensured with digital calipers (Model number NB60; Mitutoyo American Corp) [5].
Initial surface roughness and color coordinate measurements
Surface roughness of the specimens was measured by using a non-contact optical profilometer (FRT MicroProf 100 equipped with an H0 sensor; Fries Research & Technology GmbH). Three horizontal and 3 vertical linear traces, which were 1 mm apart from each other, were recorded for each specimen and averaged with a software (Mark III; Fries Research & Technology GmbH) [26]. The parameters of the profilometer were 5.5 mm of transverse length, 1000/mm of pixel density [26], 3 nm of z-axis resolution, and 0.8 mm of cut-off wavelength (Lc).
A slurry of pumice in water (Pumice fine; Benco Dental) was used for 90s (1500 rpm) [27] to polish the specimens, while a polishing paste (Fabulustre; Grobet USA) was applied for an additional 90s for fine polishing [14]. Prior to the measurements, specimens were cleaned in distilled water for 10 min by using an ultrasonic cleaner (Eltrosonic Ultracleaner 07-08; Eltrosonic GmbH) and then, dried with paper towels. Surface roughness of the specimens was then remeasured after controlling the thickness of all specimens with the same calipers. Color coordinates (L*, which corresponds to lightness; a*, which corresponds to redness; b*, which corresponds to yellowness) defined by the Commission internationale de l'éclairage (CIE) were measured by using a digital spectrophotometer (CM-26d; Konica Minolta) with an illumination aperture of 8 mm, which uses the CIE Standard (2-degree) human observer characteristics and CIE D65 illuminant in its color estimations [2]. Before each measurement, the spectrophotometer was calibrated according to the manufacturer’s recommendation. Measurements were performed over a gray background, and a saturated sucrose solution was used for optical contact. Three measurements were recorded for each specimen, and mean values of these 3 measurements were calculated as the definitive color coordinates of a specimen. Same clinician (M.S.P.) performed all color measurements in a temperature- and humidity-controlled room with day light.
Simulated brushing and coffee thermocycling
After surface roughness and color measurements, specimens were subjected to simulated brushing (Bürstmaschine linear LR1; Syndicad Engineering) by using FDA-certified tooth brushes [28] that had soft bristles [11]. Total brushing time of 20,000 cycles (40,000 strokes, each cycle considered as a linear back and forth brushing action at a frequency of 1.5 Hz) was considered to replicate complete denture cleaning of approximately 4 years, as 10,000 strokes were reported to represent 1 year of complete denture cleaning [6, 29, 30]. Six brush heads were mounted to 6 separate slots and each brush applied a vertical load of 200 g directly onto the specimen surface. A soap slurry, which replicated the complete denture cleaning medium, was homogenously prepared (T25 digital Ultra Turrax; IKA) by mixing 1 part of alkali-free ground soap (Sibonet pH 6.5; Burnus GmbH) and 3 parts of distilled water by weight [31]. Soap slurries were prepared and poured into each chamber of the brushing machine until the surface of the specimens was covered. The toothbrushes and slurry were replaced with new ones every 10,000 cycles for each specimen [32], and the test was performed at room temperature (23 °C). After brushing, the specimens were removed from the brushing machine, rinsed with distilled water, and gently air-dried.
The specimens were then subjected to 10,000 thermocycles (SD Mechatronik Thermocycler; SD Mechatronik GmbH) at 5–55 °C in a coffee solution with a dwell time of 30 s and a transfer time of 10 s [5, 33, 34]. The filtered coffee solution was prepared with a tablespoon of coffee (Intenso Roasted and Grounded; Kaffeehof GmbH) dissolved in 177 ml of water, which was freshly made in every 12 h [5, 33]. After coffee thermocycling, coffee extracts were cleaned by gently brushing the specimens 10 times with a toothpaste (Colgate Total Pro Breath Health; Colgate-Palmolive) under running water [5, 33], which was followed by ultrasonic cleaning in distilled water for 10 min (Whaledent Biosonic; Whaledent Inc).
Surface roughness and color coordinates were remeasured after brushing and coffee thermocycling, and color differences between different time intervals were calculated by using the CIEDE2000 color difference formula [5, 37], in which the parametric factors (kL, kC, and kH) were set to 1 [5, 33, 35]:
Scanning electron microscopy (SEM) analysis
Figure 2 depicts one additional sample from all test groups at different time intervals (before polishing, after polishing, and after coffee thermocycling). Scanning electron microscopy (SEM) (LEO 440, Zeiss, Cambridge, UK) images of these additional samples were also taken at different time intervals (before polishing, after polishing, and after coffee thermocycling) under × 50 magnification to analyze surface topography.
Statistical analysis
Shapiro–Wilk tests were performed to evaluate the distribution of both surface roughness and color difference data. Repeated measures analysis of variance test was used to analyze each material’s before polishing, after polishing, after brushing, and after coffee thermocycling surface roughness values, while one-way analysis of variance test was used to compare the surface roughness values within each time interval. Color difference values between after polishing and after brushing, and after polishing and after coffee thermocycling among the materials were evaluated by using one-way analysis of variance tests. All analyses were performed by using a statistical analysis software (IBM SPSS Statistics, v23; IBM Corp) at a significance level of α = 0.05. Perceptibility and acceptability of color difference values were further evaluated by the previously reported thresholds by Ren et al. (50% perceptibility threshold: 1.72 CIEDE2000 units, 50% acceptability threshold: 4.08 CIEDE2000 units) [19].
Results
Figure 3 illustrates surface roughness values of each group-time interval pair. Repeated measures analysis of variance results showed significant differences among time intervals within each material (P < 0.001). NX had the highest surface roughness before polishing (P < 0.001), whereas differences among other time intervals were nonsignificant (P ≥ 0.072). DT had the highest surface roughness before polishing (P < 0.001) and the lowest surface roughness after polishing (P ≤ 0.001). In addition, surface roughness values after coffee thermocycling were higher than those after brushing (P = 0.002). MZ had the highest surface roughness before polishing (P ≤ 0.012). After polishing and after brushing, surface roughness values of MZ were similar (P = 0.24) and lower than after coffee thermocycling (P ≤ 0.038). After polishing and after brushing, surface roughness values of CV were similar (P = 0.16) and lower than that before polishing (P < 0.001). However, surface roughness values after coffee thermocycling were similar to those at other time intervals (P ≥ 0.11). One-way analysis of variance results showed significant differences among materials within each time interval (P ≤ 0.003), except for after brushing (P = 0.091). NX had the highest (P < 0.001) and MZ had the lowest (P ≤ 0.006) surface roughness before polishing. In addition, DT had higher surface roughness than CV (P = 0.041). NX had the highest surface roughness after polishing (P ≤ 0.006), and the differences among other materials were nonsignificant (P ≥ 0.11). After coffee thermocycling, CV had the highest surface roughness (P ≤ 0.003) and the differences among other materials were nonsignificant (P ≥ 0.76).
Regardless of the material tested, SEM images before polishing had prominent irregularities with MZ having the smoothest surface. In addition, different topographies related to different manufacturing methods were also evident before polishing. However, after polishing, surfaces of all specimens were smoothened significantly, while visible lines were present on the surface of NX and CV after all procedures were completed (Fig. 4).
No significant differences were observed among color difference values of materials between after polishing and after brushing (df = 3, F = 1.075, P = 0.372), and after polishing and after coffee thermocycling (df = 3, F = 0.951, P = 0.426) (Fig. 5). Figure 6 illustrates the box-plot for color difference values’ trend between different time intervals among materials. Figure 7 shows the change in color coordinates of materials after each procedure.
Discussion
Significant differences in surface roughness were observed among time intervals within denture resins and among denture resins within each time interval. Therefore, the first null hypothesis was rejected.
Even though none of the materials tested had a surface roughness value that was similar to or lower than the previously reported threshold value of 0.2 µm [3, 5, 6, 9,10,11, 13, 14, 16] before polishing, surface roughness of all materials decreased to an acceptable range after polishing (Table S1). SEM images (Fig. 4) support this finding as the surface of each material before polishing had a rough topography, which was significantly smoother after polishing. It is also possible to observe the lamellar structure of additively manufactured specimens, particularly NX, and longitudinal lines present on MZ’s surface caused by milling burs and precision cutter before polishing. Brushing significantly increased the surface roughness of only DT as its mean surface roughness increased from 0.13 to 0.14 µm, while NX (0.21 µm) and CV (0.22 µm) had slightly higher values than 0.2 µm after brushing. However, the authors believe that such small differences may be clinically negligible; thus, it can be speculated that tested materials are resistant to long-term brushing. In addition, each material had significantly lower surface roughness values after brushing when compared with before polishing, which is in line with a previous study [9]. CV and NX had higher mean surface roughness values than 0.2 µm after coffee thermocycling (Table S1). SEM images of the surfaces after all procedures completed are also parallel with the raw surface roughness data as NX and CV had higher mean values than those of DT and MZ (Fig. 4). In addition, NX had higher surface roughness values than DT at all stages, with values before polishing being statistically significant. Even though additively manufactured resin specimens were fabricated by using their respective proprietary 3D printers with digital light processing technology, compositions of the tested materials differ from each other, which may have caused this difference. DT consists of 25– < 50%wt ethoxylated bisphenol A dimethacrylate (Bis-EMA), 25– < 50%wt aliphatic urethane dimethacrylate (UDMA) and 1– < 5%wt triethylene glycol dimethacrylate [36], while Bis-EMA comprises more than 75%wt of the NX resin mixture along with 10–20%wt methacrylate monomers, 5–10%wt silicon dioxide, 1–5%wt phosphine oxide, and < 0.1%wt titanium dioxide [37]. UDMA was reported to have higher mechanical properties than Bis-EMA [38], which may also explain the difference between additively manufactured resins. However, this interpretation needs further support with studies based on other mechanical properties of tested additively manufactured resins. Nevertheless, considering that CV, which was the only conventionally manufactured denture base resin, had the highest surface roughness values after coffee thermocycling, it can be hypothesized that CV is the material most susceptible to surface changes after brushing and coffee thermocycling among tested denture base resins.
To the authors’ knowledge, only 1 study [13] has investigated the effect of brushing on the surface roughness of additively manufactured denture base resins. Alfouzan et al. [13] compared the surface roughness of additively manufactured and conventionally manufactured denture base resins after consecutive thermocycling, brushing, and staining. Even though no significant difference was shown between materials, brushing and staining increased the surface roughness values after polishing [13]. However, in the present study, neither brushing nor coffee thermocycling affected the surface roughness of NX and CV. This contradiction between the present study and Alfouzan et al.’s [13] study may be related to the difference in tested materials and test designs. Nevertheless, considering the limited number of studies on the behavior of the surface of additively manufactured denture base resins after brushing, future studies should investigate the effect of brushing on different mechanical parameters.
Gad et al. [22] reported lower surface roughness values for additively manufactured resin compared with heat-polymerized resin after polishing. However, in the present study, while there was no difference between DT and CV, NX had higher surface roughness values than those after polishing. In Gad et al.’s [22] study, a polishing procedure and a heat-polymerized resin different than those in the present study were used, which may explain the contradicting results between these studies. In addition, differences in chemical composition of additively manufactured resins may have led to significantly different outcomes. Another study investigated the surface roughness of additively manufactured, subtractively manufactured, and conventionally manufactured denture base resins before and after polishing [24]. Similar to the present study, Di Fiore et al. [24] concluded that the differences among materials after polishing were nonsignificant. These results are also in line with other previous studies [5, 23, 25, 39]. In addition, both the present and Di Fiore et al.’s [24] studies showed that subtractively manufactured denture base resin had significantly lower baseline surface roughness values than those of other materials. This may be associated with the fact that prepolymerized PMMAs are fabricated under high pressure and temperature that may have reduced their inherent defects due to increased degree of polymerization and reduced residual monomer content [3].
Neither material type nor time interval had a significant effect on color difference values. Thus, the second null hypothesis was accepted. Among the materials tested in the present study, only MZ had a color difference value that was slightly higher than the clinically perceptible threshold (1.72 units) [19] after brushing (color difference: 1.77); tested materials had acceptable stainability (Table S2).
Alfouzan et al. [7] investigated the stainability of 2 additively manufactured denture base resins after consecutive thermocycling, brushing, and coffee immersion, comparing with conventionally manufactured resin. The authors [7] reported significant differences among the materials, conventionally manufactured resin having the lowest stainability. Thermocycling and subsequent coffee immersion of resins led to a greater color change with additively manufactured resins compared with those conventionally or subtractively manufactured. Even though the results of the present study contradict those of Alfouzan et al.’s [7] and Gruber et al.’s [2] studies, a direct comparison might be misleading given the differences in tested materials, test design, and the color difference formulae used. In addition, Alp et al. [5] have shown that subtractively manufactured denture base resin had similar color change with conventionally manufactured denture base resin after polishing and coffee thermocycling similar to those in the present study. This finding was later substantiated by Al-Qarni et al.’s [17] study as 7 days of coffee immersion had a similar effect on the stainability of subtractively manufactured and conventionally manufactured denture base resins.
Brushing slightly increased the L* values (lightness) of materials. However, coffee thermocycling decreased the L* values of all materials other than DT. NX and DT had the greatest change in a* values (redness), which decreased for NX and increased for DT throughout the procedures. MZ did not have a notable change in a* values. However, brushing increased, coffee thermocycling decreased the a* values of CV. Both brushing and coffee thermocycling increased the b* values (yellowness) of DT and CV. Brushing did not significantly affect the b* values of NX and MZ. However, coffee thermocycling decreased the b* values of NX (Fig. 7).
One of the limitations of the present study is the lack of a priori power analysis to determine the sample size, which indicates the number of specimens per group. However, significant differences were observed among test groups, and the number of specimens was based on previous studies [3, 7, 13]. The in vitro design of the present study is a limitation, considering that in vivo factors, such as saliva, were not fully simulated. One type of toothbrush and a soap slurry were used for simulated brushing and different results may be obtained with different brush, soaps, and dentifrices. During coffee thermocycling, both surfaces of the specimens were discolored. However, only polished surfaces of dentures are in contact with staining solutions clinically; thus, the results of the present study might have been amplified. In addition, only coffee was used as the staining solution, yet different staining solutions may lead to different results [7]. In addition, the present study investigated the specimens after brushing and coffee thermocycling, which can be considered as a clinical routine. However, changing the order of these tests may affect the results. Finally, 2 types of additively manufactured and 1 type of subtractively manufactured denture base resins were tested, which is a limitation. Considering that the knowledge on the properties of additively manufactured denture base resins after brushing and coffee thermocycling is limited, the results of the present study should be corroborated with future studies investigating different mechanical properties such as flexural strength and microhardness using different dentifrices, tooth brushes, and staining solutions. In addition, given the fact that the monomers in tested denture base materials might have hazardous effects, toxicity of these materials after brushing and coffee thermocycling, which may increase the leaching of these monomers, should also be investigated.
Conclusions
Based on the findings of this in vitro study, the following conclusions were drawn:
-
1.
Polishing significantly reduced the surface roughness of materials tested, while brushing and coffee thermocycling had a significant effect only on the roughness of DT.
-
2.
NX and CV had higher surface roughness than clinically acceptable threshold of 0.2 µm after all procedures were completed.
-
3.
Stainability of tested denture base materials was mostly not affected by brushing and coffee thermocycling; only MZ had a perceptible color change after brushing.
Data availability
Data will be made available on reasonable request.
References
C. Dayan, M.C. Guven, B. Gencel, C. Bural, A comparison of the color stability of conventional and CAD/CAM polymethyl methacrylate denture base materials. Acta Stomatol. Croat. 53(2), 158–167 (2019). https://doi.org/10.15644/asc53/2/8
S. Gruber, P. Kamnoedboon, M. Özcan, M. Srinivasan, CAD/CAM complete denture resins: an in vitro evaluation of color stability. J. Prosthodont. 30(5), 430–439 (2021). https://doi.org/10.1111/jopr.13246
M. Arslan, S. Murat, G. Alp, A. Zaimoglu, Evaluation of flexural strength and surface properties of prepolymerized CAD/CAM PMMA-based polymers used for digital 3D complete dentures. Int. J. Comput. Dent. 21(1), 31–40 (2018)
H. Neshandar Asli, S. Rahimabadi, Y. Babaee Hemmati, M. Falahchai, Effect of different surface treatments on surface roughness and flexural strength of repaired 3D-printed denture base: an in vitro study. J. Prosthet. Dent. 126(4), 595.e1-595.e8 (2021). https://doi.org/10.1016/j.prosdent.2021.07.005
G. Alp, W.M. Johnston, B. Yilmaz, Optical properties and surface roughness of prepolymerized poly(methyl methacrylate) denture base materials. J. Prosthet. Dent. 121(2), 347–352 (2019). https://doi.org/10.1016/j.prosdent.2018.03.001
Y.H. Chang, C.Y. Lee, M.S. Hsu, J.K. Du, K.K. Chen, J.H. Wu, Effect of toothbrush/dentifrice abrasion on weight variation, surface roughness, surface morphology and hardness of conventional and CAD/CAM denture base materials. Dent. Mater. J. 40(1), 220–227 (2021). https://doi.org/10.4012/dmj.2019-226
A.F. Alfouzan, H.M. Alotiabi, N. Labban, H.N. Al-Otaibi, S.M. Al Taweel, H.A. AlShehri, Color stability of 3D-printed denture resins: effect of aging, mechanical brushing and immersion in staining medium. J. Adv. Prosthodont. 13(3), 160–171 (2021). https://doi.org/10.4047/jap.2021.13.3.160
D.B. Sorgini, C.H. da Silva-Lovato, V.A. Muglia, R.F. de Souza, C.N. de Arruda, F.H. de Paranhos, Adverse effects on PMMA caused by mechanical and combined methods of denture cleansing. Braz. Dent. J. 26(3), 292–296 (2015). https://doi.org/10.1590/0103-6440201300028
K.M. de Freitas Pontes, J.C. de Holanda, C.S. Fonteles, B.C. de Pontes, C.H. Lovato da Silva, F.H. de Paranhos, Effect of toothbrushes and denture brushes on heat-polymerized acrylic resins. Gen. Dent. 64(1), 49–53 (2016)
M.X. Pisani, J.P. Bruhn, H.F. Paranhos, C.H. Silva-Lovato, R.F. de Souza, H. Panzeri, Evaluation of the abrasiveness of dentifrices for complete dentures. J. Prosthodont. 19(5), 369–373 (2010). https://doi.org/10.1111/j.1532-849X.2010.00592.x
L.A. Shinawi, Effect of denture cleaning on abrasion resistance and surface topography of polymerized CAD CAM acrylic resin denture base. Electron. Phys. 9(5), 4281–4288 (2017). https://doi.org/10.19082/4281
D.B. Sorgini, C.H. Silva-Lovato, R.F. de Souza, L.R. Davi, F.H. de Paranhos, Abrasiveness of conventional and specific denture-cleansing dentifrices. Braz. Dent. J. 23(2), 154–159 (2012). https://doi.org/10.1590/s0103-64402012000200011
A.F. Alfouzan, H.M. Alotiabi, N. Labban, H.N. Al-Otaibi, S.M. Al-Taweel, H.A. AlShehri, Effect of aging and mechanical brushing on surface roughness of 3D printed denture resins: a profilometer and scanning electron microscopy analysis. Technol. Health Care 30(1), 161–173 (2022). https://doi.org/10.3233/THC-212974
S. Atalay, G. Çakmak, M. Fonseca, M. Schimmel, B. Yilmaz, Effect of thermocycling on the surface properties of CAD-CAM denture base materials after different surface treatments. J. Mech. Behav. Biomed. Mater. 121, 104646 (2021). https://doi.org/10.1016/j.jmbbm.2021.104646
H. Gungor, M. Gundogdu, Z. Yesil Duymus, Investigation of the effect of different polishing techniques on the surface roughness of denture base and repair materials. J. Prosthet. Dent. 112(5), 1271–1277 (2014). https://doi.org/10.1016/j.prosdent.2014.03.023
C.M. Bollen, W. Papaioanno, J. Van Eldere, E. Schepers, M. Quirynen, D. van Steenberghe, The influence of abutment surface roughness on plaque accumulation and peri-implant mucositis. Clin. Oral Implants Res. 7(3), 201–211 (1996). https://doi.org/10.1034/j.1600-0501.1996.070302.x
F.D. Al-Qarni, C.J. Goodacre, M.T. Kattadiyil, N.Z. Baba, R.D. Paravina, Stainability of acrylic resin materials used in CAD-CAM and conventional complete dentures. J. Prosthet. Dent. 123(6), 880–887 (2020). https://doi.org/10.1016/j.prosdent.2019.07.004
M.C. Goiato, D.M. Dos Santos, G.T. Baptista, A. Moreno, A.M. Andreotti, L.C. Bannwart, S.F. Dekon, Effect of thermal cycling and disinfection on colour stability of denture base acrylic resin. Gerodontology 30(4), 276–282 (2013). https://doi.org/10.1111/j.1741-2358.2012.00676.x
J. Ren, H. Lin, Q. Huang, G. Zheng, Determining color difference thresholds in denture base acrylic resin. J. Prosthet. Dent. 114(5), 702–708 (2015). https://doi.org/10.1016/j.prosdent.2015.06.009
N.P. Sagsoz, N. Yanıkoglu, H. Ulu, F. Bayındır, Color changes of polyamid and polymetyhl methacrylate denture base materials. OJST 4(10), 489–496 (2014). https://doi.org/10.4236/ojst.2014.410066
E.A. Ayaz, B. Bağış, S. Turgut, Effects of thermal cycling on surface roughness, hardness and flexural strength of polymethylmethacrylate and polyamide denture base resins. J. Appl. Biomater. Funct. Mater. 13(3), e280-286 (2015). https://doi.org/10.5301/jabfm.5000236
M.M. Gad, S.M. Fouda, R. Abualsaud, F.A. Alshahrani, A.M. Al-Thobity, S.Q. Khan, S. Akhtar, I.S. Ateeq, M.A. Helal, F.A. Al-Harbi, Strength and surface properties of a 3D-printed denture base polymer. J. Prosthodont. 31(5), 412–418 (2022). https://doi.org/10.1111/jopr.13413
Z. Al-Dwairi, A.A. Al Haj Ebrahim, N.Z. Baba, A comparison of the surface and mechanical properties of 3D printable denture-base resin material and conventional polymethylmethacrylate (PMMA). J. Prosthodont. (2022). https://doi.org/10.1111/jopr.13491
A. Di Fiore, R. Meneghello, P. Brun, S. Rosso, A. Gattazzo, E. Stellini, B. Yilmaz, Comparison of the flexural and surface properties of milled, 3D-printed, and heat polymerized PMMA resins for denture bases: an in vitro study. J. Prosthodont. Res. (2021). https://doi.org/10.2186/jpr.JPR_D_21_00116
M. Srinivasan, N. Kalberer, P. Kamnoedboon, M. Mekki, S. Durual, M. Özcan, F. Müller, CAD-CAM complete denture resins: an evaluation of biocompatibility, mechanical properties, and surface characteristics. J. Dent. 114, 103785 (2021). https://doi.org/10.1016/j.jdent.2021.103785
C. Livas, T. Baumann, S. Flury, N. Pandis, Quantitative evaluation of the progressive wear of powered interproximal reduction systems after repeated use : an in vitro study. J. Orofac. Orthop. 81(1), 22–29 (2020). https://doi.org/10.1007/s00056-019-00200-x
O. Sahin, A. Koroglu, D. Dede, B. Yilmaz, Effect of surface sealant agents on the surface roughness and color stability of denture base materials. J. Prosthet. Dent. 116(4), 610–616 (2016). https://doi.org/10.1016/j.prosdent.2016.03.007
S. Flury, E. Diebold, A. Peutzfeldt, A. Lussi, Effect of artificial toothbrushing and water storage on the surface roughness and micromechanical properties of tooth-colored CAD-CAM materials. J. Prosthet. Dent. 117(6), 767–774 (2017). https://doi.org/10.1016/j.prosdent.2016.08.034
G.R. Goldstein, T. Lerner, The effect of toothbrushing on a hybrid composite resin. J. Prosthet. Dent. 66(4), 498–500 (1991). https://doi.org/10.1016/0022-3913(91)90511-t
C.P. Turssi, A.T. Hara, C.S. de Magalhães, M.C. Serra, A.L. Rodrigues Jr., Influence of storage regime prior to abrasion on surface topography of restorative materials. J. Biomed. Mater. Res. B Appl. Biomater. 65(2), 227–232 (2003). https://doi.org/10.1002/jbm.b.10005
E.L. Viana, G.S. Weiss, L.O. Sakae, S.H. Niemeyer, A.B. Borges, T. Scaramucci, Activated charcoal toothpastes do not increase erosive tooth wear. J. Dent. 109, 103677 (2021). https://doi.org/10.1016/j.jdent.2021.103677
T.A. Sulaiman, R.N. Camino, R. Cook, A.J. Delgado, J.F. Roulet, W.A. Clark, Time-lasting ceramic stains and glaze: a toothbrush simulation study. J. Esthet. Restor. Dent. 32(6), 581–585 (2020). https://doi.org/10.1111/jerd.12590
O. Acar, B. Yilmaz, S.H. Altintas, I. Chandrasekaran, W.M. Johnston, Color stainability of CAD/CAM and nanocomposite resin materials. J. Prosthet. Dent. 115(1), 71–75 (2016). https://doi.org/10.1016/j.prosdent.2015.06.014
M.S. Gale, B.W. Darvell, Thermal cycling procedures for laboratory testing of dental restorations. J. Dent. 27(2), 89–99 (1999). https://doi.org/10.1016/s0300-5712(98)00037-2
Y.K. Lee, Comparison of CIELAB DeltaE(*) and CIEDE2000 color-differences after polymerization and thermocycling of resin composites. Dent. Mater. 21(7), 678–682 (2005). https://doi.org/10.1016/j.dental.2004.09.005
The Saremco Website. www.saremco.ch/wp-content/uploads/2020/09/MSDS-Denturetec-en_07-2020.pdf. Accessed 13 June 2022
The NextDent Website. https://store.avadent.com/wp-content/uploads/2018/11/SDS_NextDent-Denture-3D_V2.0_20180122_EN.pdf . Accessed 25 May 2022
V.E.S. Gajewski, C.S. Pfeifer, N.R.G. Fróes-Salgado, L.C.C. Boaro, R.R. Braga, Monomers used in resin composites: degree of conversion, mechanical properties and water sorption/solubility. Braz. Dent. J. 23(5), 508–514 (2012). https://doi.org/10.1590/s0103-64402012000500007
Z.N. Al-Dwairi, K.Y. Tahboub, N.Z. Baba, C.J. Goodacre, M. Özcan, A comparison of the surface properties of CAD/CAM and conventional polymethylmethacrylate (PMMA). J. Prosthodont. 28(4), 452–457 (2019). https://doi.org/10.1111/jopr.13033
Acknowledgments
Saremco Dental AG is gratefully acknowledged for supplying the materials used in this study.
Funding
Open access funding provided by University of Bern.
Author information
Authors and Affiliations
Contributions
GÇ contributed to design and data collection. MBD contributed to drafting article and critical revision of article. MSdP contributed to data collection and drafting article. CA contributed to design and data collection. AC contributed to drafting article. MS contributed to design and critical revision of the article. BY contributed to critical revision of the article and approval of the submitted and final versions.
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Çakmak, G., Donmez, M.B., de Paula, M.S. et al. Surface roughness and stainability of new-generation denture base materials after brushing and coffee thermocycling. Journal of Materials Research (2022). https://doi.org/10.1557/s43578-022-00798-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1557/s43578-022-00798-6