Abstract
Recently infectious diseases and increasing microbial drug-resistant have caused many humorless health problems. To fill knowledge gaps and guide strategies at all levels for antimicrobials which represent a challenge and an urgent need. For these reasons, our target is developing a new effective antimicrobial drug with extended action time, multi-antimicrobial agents, low toxicity, and safe strategies. Metal–organic frameworks are promising materials for antimicrobial agents. Herein, a novel affordable Fe(III)-MOF was simply prepared via a reflux method. FE-SEM images showed an octahedral structure with sharp edges with high crystallinity and purity of Fe(III)-MOF. Under optimum conditions, the Fe(III)-MOF showed excellent antimicrobial efficiency against ± bacteria, fungus, and yeast with an inhibition zone ranging between 40–46 and 22–24 mm at a concentration of 50 and 25 μg/mL Fe(III)-MOF, respectively. As well, the mechanism of interaction is also well studied. The results open the door for the use of prepared materials as an effective and efficient antimicrobial agent.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Infectious diseases and human infections like pathogenic bacterial infection as an example, remain a major driver of morbidity/mortality and pose an immense threat to public health worldwide annually [1,2,3]. Moreover, to tackle this threat, antibiotics are widely used in large doses to treat bacterial infection conventionally [4, 5]. However, the use of administered antibiotics is often less effective or even ineffective in many cases, which can be attributed to increased drug resistance [1, 5, 6] Consequently, increasing the numbers of multidrug-resistant bacteria is considered one of the vital challenging issues that face global public health [3, 5, 7]. A report published by the “World Health Organization” (WHO) in 2017 included a list of many types of drug-resistant bacteria [2]. Therefore, traditional antimicrobial agents/drugs became not as efficient as demanded. At the same time, the antimicrobial agent’s development becomes have considerable attention and attracted many scientists around the world due to their important role in different fields such as environmental, textiles, food industry/storage, healthcare sector, medical area, treatment of infectious disease, and many technical applications [2, 8,9,10]. In this regard, a lot of reports handled several transition metals, metal oxides, metal complexes, and metallic-nanoparticles as antimicrobial agents [11,12,13,14]. Although the above materials showed efficient high antimicrobial activities, unfortunately, they sometimes have high cytotoxicity in human normal cells/tissues [3, 7]. Due to this reason, the application of some of the above materials as antimicrobial agent’s significant drawbacks.
On the other hand, MOFs and nanocomposites-based MOFs systems have demonstrated significant long period antimicrobial activity [2, 3, 7, 15,16,17]. MOFs are highly well-ordered skeletons and structures, constituting organic linker and metal center molecules [18,19,20,21,22,23,24]. The possibility of a combination of multiple-functionalities within the framework; makes these polymeric materials an ideal system for the designing of many periodic arrangements with multi-functional molecular subunits. Besides the distinct properties of these materials, they have been used in wide areas of applications [25,26,27,28]. The advantages of this category of materials as antimicrobial agents, they act as a metal ions reservoir and release these ions progressively in a prolonged period due to their synergistic action [29]. In addition, they are chemically stable and active, tunable skeletons and structure and can be controlled in their interaction with the microorganisms; so, they recently become in growth to use as a potential antimicrobial agent [7, 9, 10, 30,31,32].
Among different types of known MOFs, iron-based MOFs such as MIL-100(Fe) [33]. These types of Fe(III)-based MOFs, have high thermal/chemical stability, unsaturated metal-centers with redox properties and Lewis-acid, a facile synthesizing method, and wide availability of metal sources and linkers [34]. The above features make it one of the best candidates to be used in different applications [35, 36]. Moreover, Fe(III)-based MOFs, are more suitable for many industrial applications in comparison with Cu, Co, or Cr-based MOFs regarding the cytotoxicity of the Fe towards microorganisms and water in general, that makes Fe(III)-based MOFs become a relatively attractive MOFs material. Regarding the antimicrobial activity of Fe(III)-based MOFs in literature; herein examples of the recent progress achieved: [Fe3O(OH)(H2O)2(BDC)3]n and [Fe3O(OH)(H2O)2(BDCNH2)3]n [known as (MIL-101(Fe)) and (NH2-MIL-101(Fe)), respectively]; were investigated against many types of fungi, yeast, and bacteria; the result showed high-effective antimicrobial activities [37]. [Fe(OH)(BDC)3]n, (known as MIL-53(Fe)) also showed high efficiency as a drug carrier that improves the antibacterial activity of vancomycin with a low toxicity [38]. Besides, different applications using many composites of Fe(III)-based MOFs and in the control of the delivery process of antibacterial agents, antiviral, antiparasitic, shells encapsulating microorganisms, pathogen-mimetic, …etc. [39,40,41,42,43,44,45].
To the best of the author’s knowledge, “in this study, a novel Fe(III)-MOF was synthesized via a reflux method simply for the first time”. Several spectroscopic apparatuses were used for characterizations such as elemental analysis, SEM/EDX, UV–Vis, FT-IR, XPS, XRD, mass spectrometry, DSC/TGA, magnetometry, and photoluminescent study. The biological and antimicrobial activity of the prepared Fe(III)-MOF was investigated using the agar-standard diffusion method. Different types of microorganisms (gram-positive and negative bacteria as S. Aureus and E. coli; besides fungus and yeast as Candida spp. and A. Niger) were used in the present study. The efficiency of the prepared MOF was evaluated by measuring the inhibition zone diameter in comparison with standard antimicrobial agents. Moreover, the mechanisms of antimicrobial action are also studied.
Results and discussion
Fe(III)-MOF characterization
The Fe(.III)-MOF was prepared via a simple reaction of 1.0 mmol of NL reported by Sheta et al., [24] with 2.0 mmol ferric sulfate according to the reaction scheme (Fig. S1). A dark red-brown precipitate was obtained, the precipitate was filtered, then washed and dried well under vacuum. The elemental data of the prepared Fe(III)-MOF was in good agreement with the theoretically calculated formula for the Fe(III)-MOF monomeric unit; the Anal. Calc. (%): C44H43Fe4N7O12, (1085.24 g/mol), C, 48.70; H, 3.99; N, 9.03; found C, 49.15; H, 4.01; N, 8.98; the melting point (m. p.) was > 300 °C; and the yield was 44.7%. The structure elucidation using the acquired qualitative and quantitative microanalytical tools was discussed hereinbelow:
FE-SEM/EDX
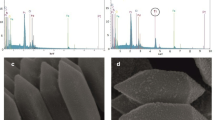
The FE-SEM images at different magnifications [Fig. 1(a)–(c)] of Fe(III)-MOF morphology appear to be an octahedral shape with dimensions in few micro-size. The results are similar to the previously reported [46]. Whereas, the EDX-analysis of Fe(III)-MOF [Fig. 1(d); and Table S1] displayed the presence of oxygen, nitrogen, carbon, and iron as a block element building of the structure. The tremendous dispersion of the concert elements through the cross-section revealed by mapping-analysis [Fig. 1(d)] confirms the Fe(III)-MOF formation. Additionally, from (Table S1) the reported EDX data was almost similar to that theoretically calculated and as well as by elemental data: C, 48.70; Fe, 20.58; N, 9.03; O, 17.69; Found: C, 49.65; Fe, 20.97; N, 9.18; and O, 20.20.
UV–Vis and FT–IR spectra
The UV–Vis spectrum and calculated bandgap energy of the Fe(III)-MOF comparable with NL were represented in Fig. 2(a) and (b), respectively. As shown in Fig. 2(a) is notice a three reflection bands at 237, 310 and 642 nm for the Fe(III)-MOF due to the “ligand–metal-charge-transfer-transitions '' (LMCT) and “intra-ligand-charge-transfers '' (n–π*/π–π*) [47]. Figure 2(b), shows the comparison between the bandgap energy values of the NL and Fe(III)-MOF, it is observed that a reduction of the values in case of Fe(III)-MOF. This can be attributed to the NL having high conjugation which led to an increase of the HOMO valence band energy which subsequently reduced the bandgap of Fe(III)-MOF. FT-IR spectrum of the Fe(III)-MOF overlay on the NL spectrum is shown in Fig. 2(c). From this [Fig. 2(c)], it can conclude that the peak between 3740 and 3230 cm−1 is due to OH of ethanol molecules and NH2 groups of Fe(III)-MOF, The sharp peaks centered at about 1634 and 1527 cm−1 are assigned to the bending of C=O, C=N, and NH, respectively. The bands between 1462 and 710 cm−1 are due to C=C and CH, respectively. The band appears at 603 cm−1 assigned to the ferric ions-oxygen coordination [ν(Fe–O)]. Whereas, the band appears at 444 cm−1 appointed to the ferric ions-nitrogen covalent bonding [ν(Fe < −N)]. The last two bands confirm the chelation of the ferric ion with NL via the O and N atoms.
Mass spectrum
The Fe(III)-MOF mass spectrum (Inside proposed fragmentation Scheme) was presented in Fig. S2. Fig. S2 shows that the ion peak is 1085.13 m/z (theoretically calculated 1085.24 g/mol) as well m/z ions peaks were fully agreed with the proposed structure molecular weight and empirical formula C44H43Fe4N7O12 which was obtained from elemental analysis. Moreover, as shown in Fig. S2 the subsequent fragmentations were in good harmony with the mass spectrum peaks.
1H/13C NMR spectra
The 1H/13C-NMR spectra of the Fe(III)-MOF were examined at room temperature in DMSO-d6 and represented in Figs. S3 and S4, respectively. The 1H-NMR spectrum (Fig. S3) ethanol molecules show three peaks; a triplet peak at 1.026, 1.040, and 1.055 ppm for (CH3), the quartet peaks for (CH2) at 3.404, 3.418, 3.431, and 3.446 ppm, besides the signal observed at 4.372 ppm assigned to NH2 protons [48, 49]. The phenyl protons peaks appeared between 6.923 and 7.946 ppm. Furthermore, the 13C-NMR spectrum (Fig. S4), of the Fe(III)-MOF confirmed the 1H-NMR spectrum that showed two peaks at 18.563 and 56.020 ppm due to CH3 and CH2 of ethanol. The signal at 30.715 ppm is due to the carbon of amine groups. The signals between 117.380 and 149.324 ppm are assigned to the carbon of phenyl rings. Finally, the peak appeared at 167.199 due to the amide carbon.
XRD analysis
The Fe(III)-MOF XRD spectrum (powder) comparable with published Fe-based MOF reports XRD patterns were represented in [Fig. 2(d)]. As showed in Fig. 2(d) the XRD peaks revealed several characteristic peaks similar to those NH2-MIL-53 type [50, 51]. The peaks are located at 2θ = 7.5, 11.1, 11.75, 12.70, 13.59, 15.21, 17.93, 25.26, 27.26 are indexed to (101), (200), (120), (110), (100), (011), (202), (020) and (220), respectively [52, 53]. The XRD patterns of the synthesized Fe-based MOF comparable with published reports showed a good agreement with the stimulated MIL-53(Fe) patterns and showed sharp peaks which confirm the crystallinity of the material [8, 54, 55].
XPS analysis
The XPS survey scans of the Fe(III)-MOF sample revealed in Fig. 3(a) and the data summarized in Table S2. The obtained data confirm the existence of (Fe, O, N, and C), also these data were in excellent harmonization that obtained from EDX analysis as well as, calculated theoretically. Moreover, The Fe 2p XPS spectrum [Fig. 3(b)] showed two satellite peaks at 715.09 for Fe(III) 2p3/2 and 728.12 eV for Fe(III) 2p1/2. As well, the spectrum showed two doublets’ peaks appeared at 710.69 eV for Fe 2p3/2 and 725.26 eV for Fe 2p1/2. These main Fe 2p peaks confirmed the oxidation state of Fe3+ in the MOF sample [38, 56]. Figure S5 shows the O 1s peaks region located at 531.14, 531.93, and 533.06 eV [57,58,59]. Fig. S6 shows the N 1s peaks region and displays two signals at 398.79, and 399.81 eV corresponding to C6H5–N–H2 and N–C=O [60, 61]. Finally, Fig. 7 displays the C 1s peaks region, which is located at 284.09, 285.34, and 288.57 eV attributed to C–N, C=O, and C=C respectively [21, 25].
BET-surface area analysis
The N2 adsorption\desorption isotherm of Fe(III)-MOF is represented in Fig. S8. The isotherm of prepared Fe(III)-MOF is of type III, according to the IUPAC classification. The BET surface area of the Fe(III)-MOF is 101.55 m2 g−1 higher than the published reports in the literature with values about 17.79 and 30.09 m2 g−1 higher than the published reports in the literatures [38, 62], respectively. Whereas, the calculated pore volume for the Fe(III)-MOF is about 0.62 cm3 g−1. Additionally, the pore size distribution curve is represented in Fig. S9. From this Figure, it can be noticed that the Fe(III)-MOF pore size was about 1.34 nm, which indicated the presence of the micropores cages in the sample (zeolitic structure).
Thermal analysis
The thermalgravimetric behavior (TGA/DSC) of the Fe(III)-MOF is presented in Fig. 3(c). The thermalgravimetric plot suggested the breakdowns of the Fe(III)-MOF through three steps. The first weight loss was about 16.97% due to the loss of four molecules of C2H5OH at 73.05 °C (theoretically calculated weight loss: 16.98%). Then the Fe(III)-MOF obeyed to decomposition in two subsequent steps of weight losses about 26 0.46, and 35.48% due to the exclusion of organic skeleton. Finally, the remaining iron residue is about 21.09% (theoretically calculated 20.58%). The thermalgravimetric behavior confirmed the other obtained data from mass, 1H and 13C NMR spectra as well obtained from EDX.
Given the physical and spectral results discussed above, it can be assumed the monomeric unit 3D structure of the Fe(III)-MOF as presented in Fig. 3(d).
Magnetic behavior and photoluminescence property of the Fe(III)-MOF
The Fe(III)-MOF magnetization curve (Fig. S10) was demonstrated the superparamagnetic behavior of the prepared Fe(III)-MOF sample, that the values of magnetization (Ms), coercivity, and remanence were 18.656 emu/g, 12.349 G, and 0.198 emu/g, respectively. Moreover, The PL-spectrum of the Fe(III)-MOF in DMSO was performed. As represented in Fig. S11, the Fe(III)-MOF exhibits a strong photoluminescence emission band (ƛmax) at 522 nm when excited at 384 nm.
Biological and antimicrobial activity studies
The antimicrobial activity of Fe(III)-MOF in vitro was investigated against gram-positive/negative bacteria and fungus/yeast. According to the experimental part, the enriched blood nutrient-agar medium was prepared and poured down to a Petri-dishes. Afterward, the fungus or bacteria were sub-cultured in the prepared media to grow microorganisms. Subsequently, the growth microorganisms were distributed over nutrient agar Petri-dishes using a sterile loop. Different families of standard antimicrobial/antifungal drugs were investigated in parallel with Fe(III)-MOF (Fig. 4). The activities of the Fe(III)-MOF were evaluated by calculating the inhibition zone diameter. The effectiveness-sensitive material is directly proportional to the diameter inhibition zone. The results of the biological activity of the Fe(III)-MOF, and the comparison of the gained data with the antifungal and antimicrobial agents used in the current study were presented in Table 1. The activities histograms for each type of microorganisms were displayed in Fig. 4. Figure 4(a), (b) for gram-positive cocci (Staph-Aureus); Fig. 4(c), (d), for gram-negative (E. Coli); and Fig. 4(e), (f) for fungus (Candida spp. and A. Niger), respectively.
The antimicrobial/antifungal activities of Fe(III)-MOF against standard antimicrobial/antifungal drugs histograms and Smartphone photos for the sensitivity evaluation of the antimicrobial and antifungal study using the agar well diffusion method for S. Aureus (a, b); E. Coli, (c, d); Candida spp., (e, f); and A. Niger (g, h), respectively.
From the obtained results and histograms, the Fe(III)-MOF showed high antimicrobial and antifungal activities against the tested bacteria and yeast. The Fe(III)-MOF showed antimicrobial activity against different tested bacteria such as gram-positive like (S. Aureus) and gram-negative like (E. Coli) with an inhibition zone ranging between 40–46 and 22–24 mm at a concentration of 50 and 25 μg/mL Fe(III)-MOF, respectively. The Fe(III)-MOF possessed potential great antifungal activity toward examined yeast strains like Candida spp. and A. Niger with inhibition zones about 35 and 38 mm, at concentrations of 50 μg/mL Fe(III)-MOF, respectively. Whereas, the inhibition zone was about 18 and 20 mm, at concentrations of 25 μg/mL Fe(III)-MOF, for both yeast, respectively. The Fe(III)-MOF showed high activity contrasted to the common drugs.
Mechanism of interaction and biological activity
Generally, the high antibacterial activity of the investigated MOF could be attributed to a variety of properties, such as the high surface area, unique shape, texture, structure, enormous porosity, the metallic plates of Fe(III) MOFs, their diffusion framework on the surface of microorganisms and the effect on the surrounding environment of the bacterial cell [63].
In the present case, as represented in Fig. 5 the suggested high antibacterial activity of Fe(III)-MOFs is due to the presence of Fe+3 on the MOF surface which led to the interaction potential between Fe+3 ions and the cell walls of microorganisms [64,65,66]. The exclusive antibacterial activity also results from the active surface of the Fe(III)-MOFs metal sites and is most probably a result of having a lot of free ions in the solution, which can promote the cooperative interaction between metal ions and bond [65]. In addition, Fe+3 ions can also bind to donor bond atoms, such as N−, O−, and S− [65, 67, 68]. These reactions depend on the coordination chemistry and are the bonding forces of –OH, –C=O, –COO–, –NH2 and –SH groups in bacterial cell walls which formed and then destroy bacterial cell walls, causing bacterial death. This activity can also be explained due to the release of Fe+3 ions from MOFs which bear the opposite charge of the metal-negative bacteria partly with the donor atoms present in the bonds (the decreasing due to the feasible delocalization of the π-electrons within chelate-ring beside the relatively partially sharing of metal-ion positive charges with the donor-site) and there is electron discrimination on the whole chelating band. This in turn increases the lipophilic-character of the Fe+3 chelate and favors its permeation across the lipid layers of bacterial membranes [15, 69,70,71,72].
Probably, the most common reason for the biotoxicity of Fe-MOF is the release of the Fe-metal from the bulk of the tire, as a cation or as small segments of the Fe-framework. An advanced topic published by Berchel et al. [73], in which they postulated that the antibacterial activity depends on the ease release of cations due to the high rigidity of the MOFs structures. Based on the Pearson theory (Pearson acid–base concept) of hard-soft Lewis-acids/bases. It suggested that the releasing of cations easily depends on the relatively stiffness of the cations “Lewis acids”, and the organic bonds “Lewis bases” of the metal framework. When soft-acid is attached to hard-base, making it more susceptible to hydrolysis and releasing the cations. In addition, the resulting electrostatic attraction is one of the reasons why killing bacteria and yeast is effective and easier. Furthermore, the effectiveness of biological activity increases with an increase in the dose (concentration) of Fe. Moreover, MOF components and their biodegradability make metal ions and sterilization ligands an auxiliary important mechanism [65].
Additionally, the ligand in MOF acts as a reservoir for metal-ions when they come into contact with the bacterial cell walls. The Fe(III)-MOF mode-of-action (a mechanism) involves hydrogen-bond formation between the NH2-groups of Fe(III)-MOF and constituents of microorganism cell-active sites. Due to the formation of these bonds a disturbance within the cell wall is generated and successively causes to damage the cytoplasmic-membrane and increases the cell-permeability which causes cell fatality. Furthermore, the functional groups of organic bonds in MOFs can react with cations in the cell, which leads to the generation of oxygen reactive species in the cytoplasm, which leads to fragmentation and modification of the DNA [15, 74,75,76].
Finally, the obtained results confirm that the Fe(III)-MOF showed higher biological/antimicrobial activity against a different class of pathogens contrasted with standard antimicrobial/antifungal agents. In addition, the results suggested that Fe(III)-MOF could be used effectively as an antimicrobial/antifungal agent and can address future medical concerns.
Conclusion
This work presents a novel affordable Fe(III)-MOF that was prepared and well-characterized. Octahedral structure with high crystallinity, purity, and sharp edges of Fe(III)-MOF was obtained. The prepared Fe(III)-MOF was evaluated as an antimicrobial agent against different types of pathogens. The Fe(III)-MOF showed antimicrobial activity against different tested bacteria such as gram-positive like (S. Aureus) and gram-negative like (E. Coli) with an inhibition zone ranging between 40–46 and 22–24 mm at a concentration of 50 and 25 μg/mL Fe(III)-MOF, respectively. The promising results of the antimicrobial study revealed that it can be one of the promising affordable future antimicrobial agents and may be used as antimicrobial for drug-resistant pathogen and simple outstanding solution for one of a vital environmental and public human health issue. Meanwhile, the vital globally reasons to prevent the advance emerging pathogens and decrease the deaths rate.
Experimental
Materials
1,2-phenylenediamine (C6H8N2); 99.5%, and ferric sulphate (Fe2(SO4)3.5H2O); 99.99% from Sigma-Aldrich were purchased. From Acros-organics, 5-aminoisophthalic acid (C8H7NO4); 98%, was purchased. The nutrient-agar medium was purchased from Himedia Lab. All the solvents or/and chemicals used in the present study were of analytical reagents grade and used as received.
Instruments and characterization
The characterization of the prepared Fe(III)-MOF was carried out using the following analytical techniques: The structure morphology was examined by using a combination of “field-emission scanning electron microscope (FE-SEM)/element mapping via spatially resolved energy-dispersive X-ray spectroscopy (EDX)” (JEOL JSM-6510LV, Japan). The Fe(III)-MOF Fourier-transform-infrared (FT-IR) and UV–Vis spectra were performed by (JASCO-FT/IR-460 spectrophotometer, and V-770-UV–vis, USA). The bandgap energy was estimated with Optbandgap-204B soft wear. Elemental analysis was done using (Costech ECS-4010- analyzer, Italy). The mass spectrum of solid Fe(III)-MOF was analyzed using a “Thermo Scientific-IQS single quadrupole spectrometer” (Thermo Scientific, USA). The 1H/13C-NMR spectra of Fe(III)-MOF in DMSO-D6 were performed with (JEOL-ECA 500II-500 MHz NMR spectrometer, Japan). The recognition of the relative crystallinity phase, and crystal size of the Fe(III)-MOF was carried out using “X-ray diffraction” (XRD) (Bruker-X-ray D8-AVANCE-diffractometer, Germany). The species and oxidation states in the Fe(III)-MOF were analyzed by an “X-ray photoelectron spectrometer” (XPS) (ThermoScientific™ K-α™, USA). The surface area of the prepared Fe(III)-MOF was measured using Quantachrome, TouchWin™ v1.2 instrument as a results of nitrogen isotherms at 77.35 K using the “Brunauer–Emmett–Teller (BET) theory”. The pore volume/size was calculated according to many methods and fitted according to the “density functional theory (DFT)” and applied to the adsorption curves. The thermal performance of the Fe(III)-MOF was investigated using “differential-scanning calorimetry/thermogravimetric analysis” (DSC/TGA) by (Universal V4.5-TA, USA). The magnetic possessions of the sample were assessed using a “Vibrating-sample magnetometer” (VSM-7400-1, USA). The photoluminescence (PL) study was performed using a (Shimadzu RF-5301PC spectro-fluorophotometer). The sample was analyzed at different excitation wavelengths and recorded the maximum emission wavelength in quartz-cuvette (1.0 cm path length). ChemBioDraw Ultra12 and Origin-8 programs were used for data analysis and drawn the structure and schemes.
Procedure
Fe(III)-MOF synthesis
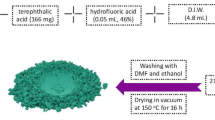
The Fe(III)-MOF was prepared according to the reaction scheme represented in Fig. S1. In 20 mL distilled-water (DW), Fe2(SO4)3.5H2O (2.0 mmol, 0.9799 g) was dissolved and then drowsily added to the organic linker previously reported by Sheta et al. [24] with stirring and then refluxed at 80 °C for 48 h. The solution color (yellowish orange) was changed to a dark red-brown precipitate which filtered off, washed, and finally dried well.
Biological and antimicrobial activity
The biological and antimicrobial activities of the Fe(III)-MOF were performed in the microbiology lab., Ministry-of-Health, Egypt, via diffusion method. Scheme 1, represented a schematic representation for the biological activity evaluation. Different types of microorganisms (bacteria: gram-positive and negative), fungus, and yeast were used in the current study; gram-positive was (S. Aureus), gram-negative was (E. coli), whereas the fungus/yeast was (Candida spp. and A. Niger). In the nutrient-agar medium, the microorganisms were sub-cultured. Nutrient-agar media was prepared according to kit-procedure: “Suspend nutrient-agar (28.0 g) in 1.0 Liter DW, then heat to boiling then sterilizing for 15 min in an autoclave at 121 °C. Left the prepared media to cool to reach 45–50 °C then poured into Petri-dishes (3 and 9 cm diameter). The media can be enhanced with 5–10% blood (calling blood-agar media), then after cooling to room temperature, the Petri-dishes were stored at 4 °C till used”. The microorganism’s culture is prepared by spreading them over each Petri dish prepared before using a sterile loop rod and incubated at 37 °C. Subsequently, after subculturing different types of bacteria, fungus, and yeast, the following step was the sensitivity evaluations of the prepared material (Fe(III)-MOF) against different families of common standard antimicrobial/antifungal agents. The microorganisms were transferred to Petri-dishes including the media, then an about 100 µL of dissolved synthesized Fe(III)-MOF (25 and 50 μmol) was added to the Petri-dishes and examined under comparable conditions with “Amikacin 500 mg, Ceftriaxone 500 mg, Ciprofloxacin 500 mg, Unasyn 375 mg, Durosiff 1 g, Flagyl 500 mg”. After incubation for 48 h at 37 °C, the inhibition zones were evaluated and measured in millimeters cautiously.
Data availability
All data generated or analysed during this study are included in this published article [and its supplementary information files].
References
G. Taubes, The bacteria fight back. Science 321, 356 (2008)
W. Nong, J. Wu, R.A. Ghiladi, Y. Guan, The structural appeal of metal–organic frameworks in antimicrobial applications. Coord. Chem. Rev. 442, 214007 (2021)
C. Pettinari, R. Pettinari, C. Di Nicola, A. Tombesi, S. Scuri, F. Marchetti, Antimicrobial MOFs. Coord. Chem. Rev. 446, 214121 (2021)
E.D. Brown, G.D. Wright, Antibacterial drug discovery in the resistance era. Nature 529(7586), 336 (2016)
V.M. Dcosta, C.E. King, L. Kalan, M. Morar, W.W.L. Sung, C. Schwarz, D. Froese, G. Zazula, F. Calmels, R. Debruyne, G.B. Golding, H.N. Poinar, G.D. Wright, Antibiotic resistance is ancient. Nature 477(7365), 457 (2011)
W. Kim, W. Zhu, G.L. Hendricks, D. Van Tyne, A.D. Steele, C.E. Keohane, N. Fricke, A.L. Conery, S. Shen, W. Pan, K. Lee, R. Rajamuthiah, B.B. Fuchs, P.M. Vlahovska, W.M. Wuest, M.S. Gilmore, H. Gao, F.M. Ausubel, E. Mylonakis, A new class of synthetic retinoid antibiotics effective against bacterial persisters. Nature 556(7699), 103 (2018)
A. Chinthamreddy, R. Karreddula, G.K. Pitchika, M.S. SurendraBabu, Synthesis, characterization of [Co(BDC)(Phen)H2O] and [Co(BDC)(DABCO)] MOFs, π.π interactions, Hirshfeld surface analysis and biological activity. J. Inorg. Organomet. Polym. Mater. 31(3), 1381 (2021)
N.A. Johari, N. Yusof, W.J. Lau, N. Abdullah, W.N.W. Salleh, J. Jaafar, F. Aziz, A.F. Ismail, Polyethersulfone ultrafiltration membrane incorporated with ferric-based metal-organic framework for textile wastewater treatment. Sep. Purif. Technol. 270(April), 118819 (2021)
M. Nakhaei, K. Akhbari, A. Phuruangrat, Synthesis and characterization of silver and copper metal–organic hybrid nanomaterials and their biological application. Colloid Polym. Sci. 299(5), 773 (2021)
W. Zhou, S. Begum, Z. Wang, P. Krolla, D. Wagner, S. Bräse, C. Wöll, M. Tsotsalas, High antimicrobial activity of metal-organic framework-templated porphyrin polymer thin films. ACS Appl. Mater. Interfaces 10(2), 1528 (2018)
Y.L. Sang, L.F. Zou, R.F. Jin, Y.H. Liu, X.S. Lin, X.H. Zhang, A series of zinc(II) and copper(II) complexes derived from 5-bromo-2((2-(dimethylamino)ethylimino)methyl)phenol: syntheses, characterization, crystal structures and antimicrobial activity. J. Coord. Chem. 74(12), 1929 (2021)
A.M. Abu-Dief, N.M. El-Metwaly, S.O. Alzahrani, F. Alkhatib, M.M. Abualnaja, T. El-Dabea, M.A.E.A.A.A. El-Remaily, Synthesis and characterization of Fe(III), Pd(II) and Cu(II)-thiazole complexes; DFT, pharmacophore modeling, in-vitro assay and DNA binding studies. J. Mol. Liq. 326, 115277 (2021)
P.M. Dahikar, Synthesis, characterization and antimicrobial activity of some metal complexes of substituted 2-hydroxybenzoinoxime. Vidyabharati Int. Interdiscip. Res. J. 11(1), 254 (2020)
G. Zhang, M.N. Nadagouda, K. O’Shea, S.M. El-Sheikh, A.A. Ismail, V. Likodimos, P. Falaras, D.D. Dionysiou, Degradation of cylindrospermopsin by using polymorphic titanium dioxide under UV-Vis irradiation. Catal. Today 224, 49 (2014)
S.M. Sheta, S.M. El-Sheikh, M.M. Abd-Elzaher, Simple synthesis of novel copper metal–organic framework nanoparticles: biosensing and biological applications. Dalt. Trans. 47, 4847 (2018)
G. Huang, Y. Yan, D. Xu, J. Wu, C. Xu, L. Fu, B. Lin, Curcumin-loaded nanoMOFs@CMFP: a biological preserving paste with antibacterial properties and long-acting, controllable release. Food Chem. 337, 127987 (2021)
A. Kumar, A. Sharma, Y. Chen, M.M. Jones, S.T. Vanyo, C. Li, M.B. Visser, S.D. Mahajan, R.K. Sharma, M.T. Swihart, Copper@ZIF-8 core-shell nanowires for reusable antimicrobial face masks. Adv. Funct. Mater. 31, 2008054 (2021)
S.M. Sheta, S.M. El, S. Mohkles, M.A. Elzaher, A.R. Wassel, A novel nano - size lanthanum metal – organic framework based on 5 - amino - isophthalic acid and phenylenediamine : photoluminescence study and sensing applications. Appl. Organomet. Chem. 33, e4777 (2019)
S.M. Sheta, S.M. El-Sheikh, M.M. Abd-Elzaher, S.R. Salem, H.A. Moussa, R.M. Mohamed, I.A. Mkhalid, A novel biosensor for early diagnosis of liver cancer cases using smart nano-magnetic metal – organic framework. Appl. Organomet. Chem. 33, e5249 (2019)
S.M. Sheta, S.M. El-sheikh, M.M. Abd-elzaher, A novel optical approach for determination of prolactin based on Pr-MOF nanofibers. Anal. Bioanal. Chem. 411, 1339 (2019)
A.S. Basaleh, S.M. Sheta, Novel advanced nanomaterial based on ferrous metal – organic framework and its application as chemosensors for mercury in environmental and biological samples. Anal. Bioanal. Chem. 412, 3153 (2020)
A.S. Basaleh, S.M. Sheta, Manganese metal – organic framework : chemical stability, photoluminescence studies, and biosensing application. J. Inorg. Organomet. Polym. Mater. 31(4), 1726 (2021)
S.M. Sheta, S.M. El-sheikh, D.I. Osman, A.M. Salem, O.I. Ali, F.A. Harraz, W.G. Shousha, M.A. Shoeib, S.M. Shawky, D.D. Dionysiou, A novel HCV electrochemical biosensor based on a polyaniline@Ni-MOF nanocomposite. Dalt. Trans. 49, 8918 (2020)
S.M. Sheta, S.M. El-Sheikh, M.M. Abd-Elzaher, M.L. Ghanem, S.R. Salem, A novel, fast, high sensitivity biosensor for supporting therapeutic decisions and onset actions for chest pain cases. RSC Adv. 9, 20463 (2019)
M. Alhaddad, S.M. Sheta, Dual naked-eye and optical chemosensor for morphine detection in biological real samples based on Cr(III) metal−organic framework nanoparticles. ACS Omega 5, 28296–28304 (2020)
S.M. Sheta, S.M. El-Sheikh, M.M. Abd-Elzaher, Promising photoluminescence optical approach for triiodothyronine hormone determination based on smart copper metal–organic framework nanoparticles. Appl. Organomet. Chem. 33, e5069 (2019)
D.I. Osman, S.M. El-Sheikh, S.M. Sheta, O.I. Ali, A.M. Salem, W.G. Shousha, S.F. El-Khamisy, S.M. Shawky, Nucleic acids biosensors based on metal-organic framework (MOF): paving the way to clinical laboratory diagnosis. Biosens. Bioelectron. 141, 111451 (2019)
S.M. El-sheikh, D.I. Osman, O.I. Ali, W. Gh, M.A. Shoeib, S.M. Shawky, S.M. Sheta, A novel Ag/Zn bimetallic MOF as a superior sensitive biosensing platform for HCV-RNA electrochemical detection. Appl. Surf. Sci. 562, 150202 (2021)
G. Wyszogrodzka, B. Marszałek, B. Gil, P. Dorozyński, Metal-organic frameworks: mechanisms of antibacterial action and potential applications. Drug Discov. Today 21(6), 1009 (2016)
S. Quaresma, P.C. Alves, P. Rijo, M.T. Duarte, V. André, Antimicrobial activity of pyrazinamide coordination frameworks synthesized by mechanochemistry. Molecules 26(7), 1904 (2021)
K. Tabatabaeian, M. Simayee, A. Fallah-Shojaie, F. Mashayekhi, M. Hadavi, Novel MOF-based mixed-matrix membranes, N-CQDs@[Zn(HCOO)3][C2H8N]/PEG, as the effective antimicrobials. J. Iran. Chem. Soc. 17(11), 2987 (2020)
I.E. Uflyand, V.A. Zhinzhilo, J.D. Bryantseva, Synthesis and study of sorption, antioxidant and antibacterial properties of MOF based on cobalt terephthalate and 1,10-phenanthroline, J. Inorg. Organomet. Polym. Mater. (2021)
N.A. Johari, N. Yusof, A.F. Ismail, F. Aziz, W.N.W. Salleh, J. Jaafar, N.H.H. Hairon, N. Misdan, The application of ferric-metal-organic framework for dye removal: a mini review. J. Adv. Res. Fluid Mech. Sci. 75(1), 68 (2020)
W. Cai, H. Gao, C. Chu, X. Wang, J. Wang, P. Zhang, G. Lin, W. Li, G. Liu, X. Chen, Engineering phototheranostic nanoscale metal-organic frameworks for multimodal imaging-guided cancer therapy. ACS Appl. Mater. Interfaces 9(3), 2040 (2017)
Y. Wu, Y. Li, J. Gao, Q. Zhang, Recent advances in vacancy engineering of metal-organic frameworks and their derivatives for electrocatalysis. SusMat 1, 66 (2021)
C. Wei, L. Tan, Y. Zhang, S. Xiong, J. Feng, Metal-organic frameworks and their derivatives in stable Zn metal anodes for aqueous Zn-ion batteries. ChemPhysMater (2021). https://doi.org/10.1016/j.chphma.2021.09.003
C. Gecgel, U.B. Simsek, M. Turabik, S. Ozdemir, Synthesis of titanium doped iron based metal-organic frameworks and investigation of their biological activities. J. Inorg. Organomet. Polym. Mater. 30(3), 749 (2020)
S. Lin, X. Liu, L. Tan, Z. Cui, X. Yang, K.W.K. Yeung, H. Pan, S. Wu, Porous iron-carboxylate metal-organic framework: a novel bioplatform with sustained antibacterial efficacy and nontoxicity. ACS Appl. Mater. Interfaces 9(22), 19248 (2017)
M. Green, Z. Liu, P. Xiang, X. Tan, F. Huang, L. Liu, X. Chen, Ferric metal-organic framework for microwave absorption. Mater. Today Chem. 9, 140 (2018)
A. Golmohamadpour, B. Bahramian, M. Khoobi, M. Pourhajibagher, H.R. Barikani, A. Bahador, Antimicrobial photodynamic therapy assessment of three indocyanine green-loaded metal-organic frameworks against Enterococcus faecalis. Photodiagn. Photodyn. Ther. 23(July), 331 (2018)
B. Claes, T. Boudewijns, L. Muchez, G. Hooyberghs, E.V. Van der Eycken, J. Vanderleyden, H.P. Steenackers, D.E. De Vos, Smart metal-organic framework coatings: triggered antibiofilm compound release. ACS Appl. Mater. Interfaces 9(5), 4440 (2017)
U.T. Uthappa, G. Sriram, O.R. Arvind, S. Kumar, G.M. Ho-Young-Jung, D.L. Neelgund, M.D. Kurkuri, Engineering MIL-100(Fe) on 3D porous natural diatoms as a versatile high performing platform for controlled isoniazid drug release, Fenton’s catalysis for malachite green dye degradation and environmental adsorbents for Pb2+ removal and dyes. Appl. Surf. Sci. 528, 146974 (2020)
P. Pei, Z. Tian, Y. Zhu, 3D printed mesoporous bioactive glass/metal-organic framework scaffolds with antitubercular drug delivery. Microporous Mesoporous Mater. 272(May), 24 (2018)
S.D. Taherzade, J. Soleimannejad, A. Tarlani, Application of metal-organic framework Nano-MIL-100(Fe) for sustainable release of doxycycline and tetracycline. Nanomaterials 7, 215 (2017)
X. Li, N. Semiramoth, S. Hall, V. Tafani, J. Josse, F. Laurent, G. Salzano, D. Foulkes, P. Brodin, L. Majlessi, N.E. Ghermani, G. Maurin, P. Couvreur, C. Serre, M.F. Bernet-Camard, J. Zhang, R. Gref, Compartmentalized encapsulation of two antibiotics in porous nanoparticles: an efficient strategy to treat intracellular infections. Part. Part. Syst. Charact. 36(3), 1 (2019)
H. Lv, H. Zhao, T. Cao, L. Qian, Y. Wang, G. Zhao, Efficient degradation of high concentration azo-dye wastewater by heterogeneous Fenton process with iron-based metal-organic framework. J. Mol. Catal. A 400, 81 (2015)
S.M. Sheta, M.M. Abd-Elzaher, S.M. El-Sheikh, A novel nano-lanthanum complex: synthesis, characterization and application as a macrofuran chemosensor in pharmaceutical, biological and environmental samples. RSC Adv. 11(16), 9675 (2021)
S.M. Sheta, M.A. Akl, E. Saad, E.R.H. El-gharkawy, A novel cerium ( III ) – isatin Schi ff base complex : application as a kidney biomarker for ultrasensitive detection of human creatinine. RSC Adv. 10, 5853 (2020)
M.A. Akl, E.R. El-gharkawy, N.A. El-mahdy, S.M. El-sheikh, S.M. Sheta, A novel nano copper complex: potentiometry, DFT and application as a cancer prostatic biomarker for the ultrasensitive detection of human PSA. Dalton Trans. 49, 15769 (2020)
N. Ahadi, S. Askari, A. Fouladitajar, I. Akbari, Facile synthesis of hierarchically structured MIL-53(Al) with superior properties using an environmentally-friendly ultrasonic method for separating lead ions from aqueous solutions. Sci. Rep. 12(1), 1 (2022)
J. Liu, X. Zou, C. Liu, K. Cai, N. Zhao, W. Zheng, G. Zhu, Ionothermal synthesis and proton-conductive properties of NH2-MIL-53 MOF nanomaterials. CrystEngComm 18(4), 525 (2016)
M. Mubashir, Y.F. Yeong, K.K. Lau, T.L. Chew, J. Norwahyu, Efficient CO2/N2 and CO2/CH4 separation using NH2-MIL-53(Al)/cellulose acetate (CA) mixed matrix membranes. Sep. Purif. Technol. 199, 140 (2018)
C.M. Fe, Q.K. Nguyen, G.M. Kuz, E.V. Khramov, R.D. Svetogorov, R.G. Chumakov, T.T. Cao, Design of metal-organic polymers MIL-53(M3+): preparation and characterization of MIL-53(Fe) and graphene oxide composite. Crystals 11, 1281 (2021)
X. Yi, W. Dong, X. Zhang, J. Xie, Y. Huang, MIL-53 ( Fe ) MOF-mediated catalytic chemiluminescence for sensitive detection of glucose. Anal Bioanal Chem. 408(30), 8805 (2016)
J. Gordon, H. Kazemian, S. Rohani, Rapid and efficient crystallization of MIL-53(Fe) by ultrasound and microwave irradiation. Microporous Mesoporous Mater. 162, 36 (2012)
Y. Gao, H. Jiang, X. Li, S.A. Khoso, G. Xiang, W. Han, Different insights into silicate rectorite modification and its role in removal of heavy metal ions from wastewater. Minerals 10, 176 (2020)
A. Barhoum, G. Van Assche, A.S.H. Makhlouf, H. Terryn, K. Baert, M.-P. Delplancke, S.M. El-Sheikh, H. Rahier, A green, simple chemical route for the synthesis of pure nanocalcite crystals. Cryst. Growth Des. 15, 573 (2015)
R.A. Geioushy, S.M. El-sheikh, A.B. Azzam, B. Ahmed, F.M. El-dars, One-pot fabrication of BiPO4/Bi2S3 hybrid structures for visible-light driven reduction of hazardous Cr (VI). J. Hazard. Mater. 381, 120955 (2020)
T.M. Khedr, S.M. El-sheikh, A.A. Ismail, D.W. Bahnemann, Highly efficient solar light-assisted TiO2 nanocrystalline for photodegradation of ibuprofen drug. Opt. Mater. (Amst) 88, 117 (2019)
Q. Zhang, G. Xia, J. Liang, X. Zhang, L. Jiang, Y. Zheng, X.-Y. Wang, NH2-MIL-53(Al) polymer monolithic column for in-tube solid-phase microextraction combined with UHPLC-MS/MS for detection of trace sulfonamides in food samples. Molecules 25, 897 (2020)
L. Huang, L. Ding, J. Zhou, S. Chen, F. Chen, C. Zhao, J. Xu, W. Hu, J. Ji, H. Xu, G.L. Liu, One-step rapid quantification of SARS-CoV-2 virus particles via low-cost nanoplasmonic sensors in generic microplate reader and point-of-care device. Biosens. Bioelectron. 171, 112685 (2021)
T. Devic, P. Horcajada, C. Serre, F. Salles, G. Maurin, B. Moulin, D. Heurtaux, G. Clet, A. Vimont, J.M. Grenéche, B. Le Ouay, F. Moreau, E. Magnier, Y. Filinchuk, J. Marrot, J.C. Lavalley, M. Daturi, G. Férey, Functionalization in flexible porous solids: effects on the pore opening and the host-guest interactions. J. Am. Chem. Soc. 132(3), 1127 (2010)
S.M. Sheta, S.M. El-Sheikh, Nanomaterials and metal-organic frameworks for biosensing applications of mutations of the emerging viruses. Anal. Biochem. 648(March), 114680 (2022)
Z. Rahmati, J. Abdi, M. Vossoughi, I. Alemzadeh, Ag-doped magnetic metal organic framework as a novel nanostructured material for highly efficient antibacterial activity. Environ. Res. 188, 109555 (2020)
M. Yang, J. Zhang, Y. Wei, J. Zhang, Recent advances in metal-organic framework-based materials for anti-staphylococcus aureus infection. Nano Res. (2022). https://doi.org/10.1007/s12274-022-4302-x
J. Liu, D. Wu, N. Zhu, Y. Wu, G. Li, Antibacterial mechanisms and applications of metal-organic frameworks and their derived nanomaterials. Trends Food Sci. Technol. 109, 413 (2021)
K.L. Haas, K.J. Franz, Application of metal coordination chemistry to explore and manipulate cell biology. Chem. Rev. 109(10), 4921 (2009)
Z. Ma, F.E. Jacobsen, D.P. Giedroc, Coordination chemistry of bacterial metal transport and sensing. Chem. Rev. 109(10), 4644 (2009)
L.A. Mermel, J.M. Cartony, P. Covington, G. Maxey, D. Morse, Methicillin-resistant Staphylococcus aureus colonization at different body sites: a prospective, quantitative analysis. J. Clin. Microbiol. 49(3), 1119 (2011)
G.R. Sampedro, A.C. De Dent, R.E.N. Becker, B.J. Berube, M.J. Gebhardt, H. Cao, J.B. Wardenburg, Targeting Staphylococcus aureus α-toxin as a novel approach to reduce severity of recurrent skin and soft-tissue infections. J. Infect. Dis. 210(7), 1012 (2014)
R. Li, T. Chen, X. Pan, Metal-organic-framework-based materials for antimicrobial applications. ACS Nano 15(3), 3808 (2021)
M. Muthukumar, P. Viswanathamurthi, Spectral, catalytic, and antifungal studies of ruthenium(II) chalcone complexes. J. Coord. Chem. 63(7), 1263 (2010)
M. Berchel, T. Le Gall, C. Denis, S. Le Hir, F. Quentel, C. Elléouet, T. Montier, J.M. Rueff, J.Y. Salaün, J.P. Haelters, G.B. Hix, P. Lehn, P.A. Jaffrès, A silver-based metal-organic framework material as a “reservoir” of bactericidal metal ions. New J. Chem. 35(5), 1000 (2011)
N. Vamsikrishna, M.P. Kumar, G. Ramesh, N. Ganji, S. Daravath, DNA interactions and biocidal activity of metal complexes of benzothiazole Schiff bases : synthesis, characterization. J. Chem. Sci. 129(5), 609 (2017)
M.D. Pulido, A.R. Parrish, Metal-induced apoptosis: mechanisms. Mutat. Res. 533(1–2), 227 (2003)
A.M. Abu-Dief, F.M.M. Alrashedee, K.M. Emran, H.A. Al-Abdulkarim, Development of some magnetic metal–organic framework nano composites for pharmaceutical applications. Inorg. Chem. Commun. 138, 109251 (2022)
Acknowledgments
This work was supported by the financial support of the Science and Technology Development Fund (STDF) Foundation of the Project No. (37068).
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sheta, S.M., Salem, S.R. & El-Sheikh, S.M. A novel Iron(III)-based MOF: Synthesis, characterization, biological, and antimicrobial activity study. Journal of Materials Research 37, 2356–2367 (2022). https://doi.org/10.1557/s43578-022-00644-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/s43578-022-00644-9