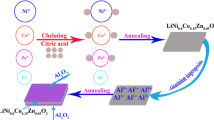
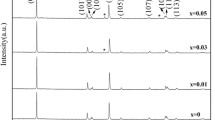
Abstract
The ZrO2-coated LiNi0.4Co0.2Mn0.4O2 with significantly improved electrochemical performance was successfully synthesized by molten salt method. The ZrO2-coated cathode material exhibited a significantly improved cycle performance and the rate performance. The 0.5 wt% ZrO2 coating material showed an excellently electrochemical cycleability and a high rate capability in the voltage range of 2.5–4.6 V. The capacity retention of 0.5 wt% ZrO2 coating material was 89.86% after 100 cycles at 0.5 C rate, which was much higher than that of raw material (82.02%). The discharge specific capacity was 106.13 mAh·g−1 after once activation at 0.1 C and 10 charge–discharge cycles at 5 C, which was much higher than that of raw material (81.71 mAh·g−1). The charge transfer impedance of coated material at high cut off voltage (4.6 V), as indicated by EIS results, was effectively suppressed, which precisely explained the distinct improvement of electrochemical performances.
Graphical abstract











Similar content being viewed by others
Data availability
We declare that all data generated or analysed during this study are included in this published article [and its supplementary information files]. And the datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
G. Zubi, R. Dufo-Lopez, M. Carvalho, G. Pasaoglu, The lithium-ion battery: State of the art and future perspectives. Renew. Sust. Energ. Rev. 89, 292–308 (2018). https://doi.org/10.1016/j.rser.2018.03.002
E. Nakamura, A. Kondo, M. Matsuoka, T. Kozawa, M. Naito, H. Koga, H. Iba, Preparation of LiCoO2/Li1.3Al0.3Ti1.7(PO4)(3) composite cathode granule for all-solid-state lithium-ion batteries by simple mechanical method, Adv. Powder Technol., 27 (2016) 825–829. https://doi.org/10.1016/j.apt.2015.10.013
C.S. Yoon, D.W. Jun, S.T. Myung, Y.K. Sun, Structural Stability of LiNiO2 Cycled above 4.2 V, ACS Energy Lett., 2 (2017) 1150–115. https://doi.org/10.1021/acsenergylett.7b00304
L.Q. Mu, X. Feng, R.H. Kou, Y. Zhang, H. Guo, C.X. Tian, C.J. Sun, X.W. Du, D. Nordlund, H.L.L. Xin, F. Lin, Deciphering the Cathode-Electrolyte Interfacial Chemistry in Sodium Layered Cathode Materials. Adv. Energy Mater. 8, 12 (2018). https://doi.org/10.1002/aenm.201801975
D.K. Kim, P. Muralidharan, H.W. Lee, R. Ruffo, Y. Yang, C.K. Chan, H. Peng, R.A. Huggins, Y. Cui, Spinel LiMn2O4 Nanorods as Lithium Ion Battery Cathodes. Nano Lett. 8, 3948–3952 (2008). https://doi.org/10.1021/nl8024328
T. Nakamura, Y. Miwa, M. Tabuchi, Y. Yamada, Structural and surface modifications of LiFePO4 olivine particles and their electrochemical properties, J. Electrochem. Soc., 153 (2006) A1108-A1114. https://doi.org/10.1149/1.2192732
Z.L. Liu, A.S. Yu, J.Y. Lee, Synthesis and characterization of LiNi1-x-yCoxMnyO2 as the cathode materials of secondary lithium batteries. J. Power Sources 81, 416–419 (1999). https://doi.org/10.1016/S0378-7753(99)00221-9
Z.H. Lu, D.D. MacNeil, J.R. Dahn, Layered Li NixCo1-2xMnx O-2 cathode materials for lithium-ion batteries. Electrochem. Solid State Lett. 4, A200–A203 (2001). https://doi.org/10.1149/1.1413182
H.B. Rong, M.Q. Xu, B.Y. Xie, W.Z. Huang, X.L. Liao, L.D. Xing, W.S. Li, Performance improvement of graphite/LiNi0.4Co0.2Mn0.4O2 battery at high voltage with added Tris (trimethylsilyl) phosphate, J. Power Sources, 274 (2015) 1155–1161. https://doi.org/10.1016/j.jpowsour.2014.10.123
J. Li, J.M. Zheng, Y. Yang, Studies on storage characteristics of LiNi0.4Co0.2Mn0.4O2 as cathode materials in lithium-ion batteries, J. Electrochem. Soc., 154 (2007) A427-A432. https://doi.org/10.1149/1.2711068
Z. Chen, G.T. Kim, D. Bresser, T. Diemant, J. Asenbauer, S. Jeong, M. Copley, R.J. Behm, J. Lin, Z.X. Shen, S. Passerini, MnPO4-Coated Li(Ni0.4Co0.2Mn0.4)O-2 for Lithium(-Ion) Batteries with Outstanding Cycling Stability and Enhanced Lithiation Kinetics, Adv. Energy Mater., 8 (2018) 13. https://doi.org/10.1002/aenm.201801573
Q.C. Chen, G.J. Yan, L.M. Luo, F. Chen, T.F. Xie, S.C. Dai, M.L. Yuan, Enhanced cycling stability of Mg-F co-modified LiNi0.6Co0.2Mn0.2-yMgyO2-zFz for lithium-ion batteries, Trans. Nonferrous Met. Soc. China, 28 (2018) 1397–1403. https://doi.org/10.1016/S1003-6326(18)64778-8
Q.Y. Chen, C.Q. Du, D.Y. Qu, X.H. Zhang, Z.Y. Tang, Synthesis and characterization of Zr-doped LiNi0.4Co0.2Mn0.4O2 cathode materials for lithium ion batteries, RSC Adv., 5 (2015) 75248–75253. https://doi.org/10.1039/C5RA14376D
C.J. Lv, Y. Peng, J. Yang, X.C. Duan, J.M. Ma, T.H. Wang, Electrospun Nb-doped LiNi0.4Co0.2Mn0.4O2 nanobelts for lithium-ion batteries, Inorg. Chem. Front., 5 (2018) 1126–1132. https://doi.org/10.1039/C7QI00811B
G. Kobayashi, Y. Irii, F. Matsumoto, A. Ito, Y. Ohsawa, S. Yamamoto, Y.T. Cui, J.Y. Son, Y.C. Sato, Improving cycling performance of Li-rich layered cathode materials through combination of Al2O3-based surface modification and stepwise precycling. J. Power Sources 303, 250–256 (2016). https://doi.org/10.1016/j.jpowsour.2015.11.014
S.C. Dai, M.L. Yuan, L. Wang, L.M. Luo, Q.C. Chen, T.F. Xie, Y.P. Li, Y.T. Yang, Ultrathin-Y2O3-coated LiNi0.8Co0.1Mn0.1O2 as cathode materials for Li-ion batteries: Synthesis, performance and reversibility, Ceram. Int., 45 (2019) 674–680. https://doi.org/10.1016/j.ceramint.2018.09.227
Q.C. Chen, L.M. Luo, L. Wang, T.F. Xie, S.C. Dai, Y.T. Yang, Y.P. Li, M.L. Yuan, Enhanced electrochemical properties of Y2O3-coated-(lithium-manganese)-rich layered oxides as cathode materials for use in lithium-ion batteries. J. Alloy. Compd. 735, 1778–1786 (2018). https://doi.org/10.1016/j.jallcom.2017.11.362
M.R. Laskar, D.H.K. Jackson, S.Z. Xu, R.J. Hamers, D. Morgan, T.F. Kuech, Atomic Layer Deposited MgO: A Lower Overpotential Coating for Li Ni0.5Mn0.3Co0.2 O-2 Cathode, ACS Appl. Mater. Interfaces, 9 (2017) 11231–11239. https://doi.org/10.1021/acsami.6b16562
J.Z. Kong, C. Ren, G.A. Tai, X. Zhang, A.D. Li, D. Wu, H. Li, F. Zhou, Ultrathin ZnO coating for improved electrochemical performance of LiNi0.5Co0.2Mn0.3O2 cathode material, J. Power Sources, 266 (2014) 433–439. https://doi.org/10.1016/j.jpowsour.2014.05.027
G.M. Song, Y. Wu, G. Liu, Q. Xu, Influence of AlF3 coating on the electrochemical properties of LiFePO4/graphite Li-ion batteries. J. Alloy. Compd. 487, 214–217 (2009). https://doi.org/10.1016/j.jallcom.2009.06.153
F. Wu, X.X. Zhang, T.L. Zhao, L. Li, M. Xie, R.J. Chen, Multifunctional AlPO4 Coating for Improving Electrochemical Properties of Low-Cost Li Li0.2Fe0.1Ni0.15Mn0.55 O-2 Cathode Materials for Lithium-Ion Batteries, ACS Appl. Mater. Interfaces, 7 (2015) 3773–3781. https://doi.org/10.1021/am508579r
S.K. Hu, G.H. Cheng, M.Y. Cheng, B.J. Hwang, R. Santhanam, Cycle life improvement of ZrO2-coated spherical LiNi1/3Co1/3Mn1/3O2 cathode material for lithium ion batteries. J. Power Sources 188, 564–569 (2009). https://doi.org/10.1016/j.jpowsour.2008.11.113
J.Z. Kong, S.S. Wang, G.A. Tai, L. Zhu, L.G. Wang, H.F. Zhai, D. Wu, A.D. Li, H. Li, Enhanced electrochemical performance of LiNi0.5Co0.2Mn0.3O2 cathode material by ultrathin ZrO2 coating, J. Alloy. Compd., 657 (2016) 593–600. https://doi.org/10.1016/j.jallcom.2015.10.187
Z.Y. Wang, E.Z. Liu, L.C. Guo, C.S. Shi, C.N. He, J.J. Li, N.Q. Zhao, Cycle performance improvement of Li-rich layered cathode material Li Li0.2Mn0.54Ni0.13Co0.13 O-2 by ZrO2 coating, Surf. Coat. Technol., 235 (2013) 570–576. https://doi.org/10.1016/j.surfcoat.2013.08.026
S.T. Myung, K. Izumi, S. Komaba, H. Yashiro, H.J. Bang, Y.K. Sun, N. Kumagai, Functionality of oxide coating for Li Li0.05Ni0.4Co0.15Mn0.4 O-2 as positive electrode materials for lithium-ion secondary batteries, J. Phys. Chem. C, 111 (2007) 4061–4067. https://doi.org/10.1021/jp0674367
K.M. Shaju, G.V.S. Rao, B.V.R. Chowdari, Performance of layered Li(Ni1/3Co1/3Mn1/3)O-2 as cathode for Li-ion batteries. Electrochim. Acta 48, 145–151 (2002). https://doi.org/10.1016/S0013-4686(02)00593-5
Z.C. Liu, H.H. Zhen, Y. Kim, C.D. Liang, Synthesis of LiNiO(2) cathode materials with homogeneous Al doping at the atomic level. J. Power Sources 196, 10201–10206 (2011). https://doi.org/10.1016/j.jpowsour.2011.08.059
S.N. Kwon, M.Y. Song, H.R. Park, Electrochemical properties of LiNiO2 substituted by Al or Ti for Ni via the combustion method. Ceram. Int. 40, 14141–14147 (2014). https://doi.org/10.1016/j.ceramint.2014.05.149
J.G. Li, L. Wang, Q. Zhang, X.M. He, Electrochemical performance of SrF2-coated LiNi1/3Co1/3Mn1/3O2 cathode materials for Li-ion batteries. J. Power Sources 190, 149–153 (2009). https://doi.org/10.1016/j.jpowsour.2008.08.011
S.J. Shi, Y.J. Mai, Y.Y. Tang, C.D. Cu, X.L. Wang, J.P. Tu, Preparation and electrochemical performance of ball-like LiMn0.4Ni0.4Co0.2O2 cathode materials, Electrochim. Acta, 77 (2012) 39–46. https://doi.org/10.1016/j.electacta.2012.05.110
J.M. Zheng, D.R. Zhu, Y. Yang, Y.S. Fung, The effects of N-methyl-N-butylpyrrolidinium bis(trifluoromethylsulfonyl)imide-based electrolyte on the electrochemical performance of high capacity cathode material Li Li0.2Mn0.54Ni0.13Co0.13 O-2, Electrochim. Acta, 59 (2012) 14–22. https://doi.org/10.1016/j.electacta.2011.09.069
R. Guo, P.F. Shi, X.Q. Cheng, L. Sun (2009) Effect of ZnO modification on the performance of LiNi0.5Co0.25Mn0.25O2 cathode material. Electrochim. Acta 54: 5796–5803. https://doi.org/10.1016/j.electacta.2009.05.034
Acknowledgments
This work was supported by the Major Science and Technology Research of Guangxi Department of Funded Projects (Grant No. 1114022-15).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the position presented in, or the review of, the manuscript entitled.
Rights and permissions
About this article
Cite this article
Liu, J., Yuan, M., Xie, T. et al. Ultrathin-ZrO2-coated LiNi0.4Co0.2Mn0.4O2 cathode material for Li-ion batteries: Synthesis and electrochemical performance. Journal of Materials Research 37, 1019–1029 (2022). https://doi.org/10.1557/s43578-022-00511-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/s43578-022-00511-7