Abstract
Here, we employ first-principles methods to expand the set of functional materials known as ferroelectrics and antiferroelectrics. We use known compounds, whose properties have previously been overlooked, as the springboard for new materials. We first develop methodology to search for polar instabilities in known nonpolar and antipolar compounds and then use this technique to identify new members of the A2BX3 family. This methodology identities new Pb-free oxides and chalcogenides, with a wide range of band gaps, to be used as solid-state photovoltaics. Finally, we perform a cursory evaluation of how compositional tuning of oxide materials can adjust the difference in energy between ground and metastable states, to assess the likelihood of polarization switching. In total, we report on a targeted sets of new materials to synthesize and design routes to obtain ferroelectrics and antiferroelectrics with energetic barriers amenable to switching with an electric field.
Graphic abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
To augment experimental endeavors, computational investigations of a variety of materials have provided atomistic insights into a wealth of new compounds that display switchable polarizations, specifically solid-state ferroelectrics (FEs) and antiferroelectrics (AFEs) [1,2,3,4,5,6,7,8,9,10]. FE materials demonstrate a switchable polarization, most times driven by an applied electric field, and through a higher-symmetry nonpolar paraelectric intermediate state. FEs also have a ground state that can be classified in crystallographic space groups that allow for polarization, so they are found within the ten polar crystal classes 6 mm, 6, 3 mm, 3, 4 mm, 4, mm2, m, 2, and 1 [11, 12]. AFEs, though similar in composition, are different; they have an antipolar ground state that is close in energy to a metastable FE state and can exhibit multiple polarization states [13]. The excitement in finding new examples of these types of functional materials is apparent in the last decade of research which has demonstrated that FEs are capable of energy production [14,15,16], and AFEs are capable of energy storage [17,18,19,20].
A few common examples of solid-state FEs include the perovskites [21,22,23,24,25,26,27], Ruddlesden-Popper phases [28, 29], Aurivillius and Dion Jacobson compounds [30,31,32,33,34], boracites [35, 36], tellurides [37, 38], and K2SeO4-type materials [39, 40]. Solid-state AFEs also include the perovskites [41,42,43,44,45,46], as well as sulfoaluminates [47], ZrO2, and HfO2 [48, 49], orthorhombic ABC semiconductors [2, 7], Bi2SiO5 [50, 51], and tungsten bronzes [52]. There are a preponderance of example FEs and AFEs that include oxides, sulfides, and selenides, and multiple examples where a variety of experimental techniques such as doping, film growth, strain engineering, confinement, and compositional tuning has resulted in the stabilization of new phases and resulted in enhanced material performance [17, 18, 43, 49, 53].
To complement both experimental techniques and the re-examination and development of new ferroic phase transitions theories [13, 54, 55], we focus on new example families of potentially functional materials to investigate as candidate FEs and AFEs. In prior work, we combined density functional theory (DFT) and crystallographic database mining to establish a family of known compounds as materials whose functional properties have previously gone overlooked [56]. This family has a general chemical formula of A2BX3 where A is a 1+ cation (Li, Na, K, Rb, Cs, Cu, Ag), B is a 4+ cation (Ti, Zr, C, Si, Ge, Sn, Pb), and X is a 2- anion (O, S, Se). While the compositions contain similar elements to conventional perovskite oxides and the other examples listed in the previous paragraph (K, Cs, Ti, Zr, O, S, etc.), the A2BX3 family of materials has markedly different coordination environments. For example, the B-site is within a fivefold coordinate square pyramid, where the BX5 pyramids are edge-sharing units in a 1D network. The rotation of these units aligned or anti-aligned creates either polar or antipolar states, as depicted in Fig. 1. The structures in Fig. 1 are classified in crystallographic space groups Cmcm (#63), Pnma (#62), and Cmc21 (#36), which we define as being nonpolar, antipolar, and polar, respectively. Compositions such as K2PbO3 and Rb2PbO3 were reported in space groups Pnma and Cmc21 [57,58,59], while Cs2PbO3 was reported in space groups Cmcm and Cmc21 [59, 60]. When members of a materials family are reported in multiple space groups, this is a strong indication that the energetic barriers between nonpolar, antipolar, and polar states might be low. If so, then the family serves as an ideal model system for analysis from a computational materials design perspective.
Shown here are the 3 primary structure types of the orthorhombic A2BX3 investigated here. The antipolar (lower left-hand side) and polar (lower right-hand side) structural variants are symmetry related to a nonpolar parent structure. Each A-site is depicted as a solid black sphere, and each B-site is depicted as a light blue sphere inside of a BX5 square pyramid composed, whereas the X-sites are depicted as red spheres.
Prior to our initial investigation, there were few reports of how any of the known structures might be related to each other [61,62,63] and to the best of our knowledge none that described any member of the family as a candidate FE or AFE. Here, we more fully map out the potential energy landscape of A2BX3 family of materials and further develop their novel structure–property relationships. First, we develop symmetry-based methodology to search for polar instabilities in nonpolar and antipolar compounds and then use these techniques to examine potentially new structure types of stoichiometry A2BX3. Second, we use DFT to identify new examples of oxide and chalcogenide materials with a wide range of band gaps using this method, and finally, we perform a cursory evaluation of how compositional tuning of known materials can adjust ∆E, the difference in energy between ground and metastable states. In total, we (i) report on targeted sets of new materials to synthesize, (ii) design routes to obtain polar and antipolar ground-state structures, and (iii) better understand their spectrum of (meta)stable states.
Results and discussion
Our prior work predicts that the A2BX3 family of materials contain AFE and FE members whose properties have previously been overlooked. The AFEs have a computed antipolar ground state, that is within < 10 meV/fu of a polar metastable state, and are the previously synthesized compounds K2SnO3, K2PbO3, Rb2PbO3, K2TiO3, K2ZrO3, and K2TiS3. One composition, predicted to be FE, Cs2PbO3, has a polar ground state that is within < 10 meV/fu of an antipolar metastable state [56]. The barrier to switch between the polar and antipolar states is approximated as ∆E1, which here is the DFT-computed difference in total energy between antipolar and polar structures, or the AFE to FE switching barrier. Another metric of use is the computed FE barrier to switch between polar and nonpolar states, which here is labeled ∆E2. A depiction of the relationships between nonpolar, antipolar, and polar A2BX3 structures and their energetics is shown in Fig. 1. The values of ∆E1 are used to assess the likelihood of switching between polar and antipolar states, and the values of ∆E2 are used to assess the likelihood of switching between polar and nonpolar states. To compare to known FE oxides, the DFT-computed ∆E2 would be ~ 20 and 200 meV/f.u. for BaTiO3 and PbTiO3, respectively [21]. The larger value of 200 ~ meV/f.u. has been considered the upper limit of FE switching in prior DFT studies [1, 64].
The displacement patterns of cations and anions in the antipolar and polar structures relative to the high symmetry nonpolar parent structure, as shown in Fig. 1, can be illustrated using irreducible representations (irreps) of the D2h point group. The antipolar distortion can be described using the amplitude of the Y2− irrep and the polar distortion can be described using the amplitude of the Γ2− irrep. Each of the displacement patterns is characterized primarily as a series of tilts within the 1D network of edge-sharing square-pyramidal BX5 units, while the A-sites remain almost stationary with respect to the parent structure. Also of importance is that the polar and antipolar structures are related by a 1st order phase (discontinuous) transition, where only 1/2 of the BX5 square pyramids are tilted in the same direction in either structure. In Fig. 1 these are the BX5 square pyramids with a black A-site depicted in front of them. The other BX5 square pyramids are in an opposing orientation so ∆E1 is a measure of the energy required to orient all BX5 square pyramids in the same direction.
It should be noted that the polar distortions in this work do not result in the known Na2SiO3 structure type, but a yet to be reported structure type in the same crystallographic space group. The B-site in the Na2SiO3 structure type are fourfold coordinate and this difference in structure types is explored in Ref. [56]. The present study serves to identify (a) a complete set of polar metastable states for a proposed set of AFEs, (b) new semiconductive members of this family that are yet to be reported, and (c) compositionally tuned structural variants where ∆E values changes as a function of atomistic identity.
Identifying metastable polar states
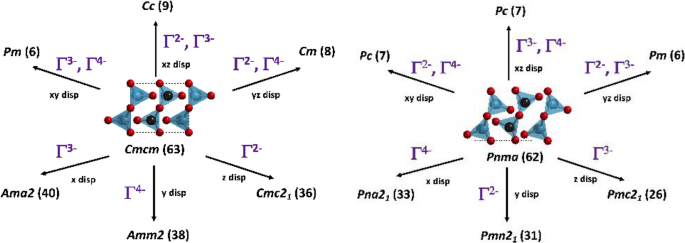
Inspired by recent investigations into the interplay between AFEs and improper FEs [65] we aim to develop a systematic route to search for new examples of FEs and improper FEs from AFEs. We start with harvesting known nonpolar and antipolar A2BX3 structure types from the ICSD and (i) create polar displacements in 1 Cartesian direction, resulting in 1 ungerade irrep, or (ii) couple polar displacements in 2 Cartesian directions, resulting in 2 ungerade irreps. The series of polar space groups that result from applying this procedure to select A2BX3 structures is shown in Fig. 2. Not depicted are the nonpolar modes that could result in a trilinear coupling scheme as observed in improper FEs. For example, polar displacements in both Cartesian x and z directions from the Cmcm structure would result in a structure of Cc (#9) symmetry, while another route to obtain Cc symmetry would be to couple polar mode Γ2− to nonpolar mode Γ4+, allowing for a polar mode Γ3− to condense in after full-cell relaxation. This is what occurred for Cs2PbO3 in Ref. [56]. Here we would induce both polar irreps simultaneously and then let the structure fully relax. This search strategy is not based on the specific symmetry considerations of specific structure types, but a generalizable procedure that can be implemented for any nonpolar or antipolar structure type. The results of this procedure on a test set of related compounds can also yield insights into the complete set of polar distortions one might observe in experiment for ternary or quaternary tuned compositions.
Our test set includes 3 oxide compounds that we predict to have an antipolar ground state, as well as a DFT-predicted metastable polar state close in energy. K2TiO3, K2PbO3, and Rb2PbO3 and were chosen to observe how sets of polar distortions would behave when the electronic state of the B-sites (d0 vs. main group cations) and size of the A-site (as K and Rb) vary. We selectively induce polar displacements in both the nonpolar parent structure and antipolar ground state to obtain the set of potential polar distortions that could be engineered using experimental parameters such as strain, compositional tuning, and epitaxial growth to stabilize a FE state. Here we do not include displacements from the A2BX3 structure types found in C2/c symmetry, previously investigated in Ref. [56], as this nonpolar structure type was rarely found to be close in energy to the antipolar ground state or metastable polar state. The complete set of irreps that result from the induced atomic displacements is illustrated in Fig. 2. If polar displacements are made along one direction this results in a polar orthorhombic structure (Amm2, Cmc21, Pna21, etc.) and if polar displacements are made along two directions this results in polar monoclinic structures (Cc, Pc, Pm, etc.) The resulting structures are then used as the input for our DFT relaxations to see if any polar distortions are stable (or metastable) in our test set.
We find for K2TiO3, K2PbO3, Rb2PbO3 that only 1 polar displacement pattern, described using the irrep Γ2−, does not relax back to high symmetry space group Cmcm. This is the polar phase depicted in Fig. 1 when displacements are made along the z-axis. For our test cases we find that the resulting structure can be classified in crystallographic space group Cmc21 and is representative of a new polar structure type. This polar structure is only 1, 3, and 3 meV/fu higher in energy than the predicted antipolar ground states of K2TiO3, K2PbO3, and Rb2PbO3, respectively. This small ΔE1 could explain why the structures of K2PbO3 and Rb2PbO3 were previously assigned to both polar and antipolar structure types [57,58,59]. The % change in volume corresponding to a transition between antipolar and polar phases is 0.18, 0.41, and 0.39% for K2TiO3, K2PbO3, and Rb2PbO3, respectively, and the orientation of the edge-sharing BX5 square pyramids in the polar phase can be easily switched relative to the high symmetry nonpolar parent structure (Cmcm symmetry).
We believe that the metastable polar structures of the test set are FE, making the antipolar ground state an AFE. All polar displacements from antipolar space group Pnma relax back to Pnma, indicating that the for the test set the antipolar irrep Y2− does not couple to any polar displacements. While the compositions K2TiO3, K2PbO3, and Rb2PbO3 display only 1 metastable polar state (classified in space group Cmc21) this procedure can be used to map out the potential energy landscape of new members of the A2BX3 family and be incorporated into future high-throughput studies.
Creating new members of the family
The electronic band structures of the antipolar ground-state compounds investigated in the previous subsection, the previously synthesized oxides K2TiO3, K2PbO3, and Rb2PbO3, are plotted in Fig. 3, next to a projected density of states (PDOS) for each compound. It should be noted that semi-local GGA methods tend to underestimate Egap values, and here we use the computed Egap values as a qualitative guide to better understand how atomistic composition affects the electronic structure. The band gap (Egap) of K2TiO3 in Fig. 3a is 3.66 eV, much larger than for K2PbO3 and Rb2PbO3, which are 1.48 and 1.50 eV, respectively, as shown in Fig. 3b and c. A comparative analysis of the PDOS shows that this large difference in Egap is primarily caused by the structures with B = Pb having a band centered at approximately 2.5 eV. This character of this band is mostly composed of contributions from the Pb 6s-orbital and O 2p-orbital. The overlap of the Pb 6s and O 2p orbitals suggest that this band comes from the bonding interaction of the Pb and O, since they are in close contact in square-pyramidal geometry. Notably, this band structure feature is nearly identical to the lowest unoccupied orbital (LUMO) of K2TiO3, but closer to the Fermi level (EF, set to 0 here). This demonstrates that substitutions of Pb for Ti can lower the band energy in select compositions by adjust the electronic states in a qualitative sense of having a B-site with either empty (Ti) or filled (Pb) d-states. As expected, the occupied states of all 3 structures have predominantly O 2p character. This is because O is highly electronegative, and therefore electrons are pulled toward the O atoms within the structure, as observed in oxides found in the perovskite and delafossite families.
While K2PbO3 and Rb2PbO3 have band gaps of 1.48 and 1.50 eV, respectively, K2TiO3 has Egap of 3.66 eV, just out of the range of semiconductors. To examine Pb-free materials and decrease the Egap of materials in this family with K and Ti, we can switch our focus from the effects of changing A and B-site cations to the effects of changing the anion X from oxygen to sulfur and selenium. S and Se are less electronegative than O, form more covalent/less ionic bonds than O and tend to decrease Egap. DFT examples where S and Se anion substitutions for O can decrease Egap include BaZrS3 [66], BaZrSe3 [67], CaZrS3 [68], PbTiO3−xSx [69] and the sets of materials identified in Ref. [70]. The Egap values presented in Ref. [70] also include materials that have been experimentally prepared (CaZrS3, BaZrS3, Ba3ZrS7, etc.) with Egap lower than their oxide counterparts, qualitatively consistent with original DFT reports.
Crystallographic database mining shows that K2TiO3 [62], K2TiS3 [71], K2ZrO3 [72], Cs2ZrO3 [73], and Cs2ZrSe3 [61] compounds are known, but combinations with Rb are not yet reported, nor are select S and Se compositions. Here we use the procedures outlined in the previous subsection to map out the potential energy landscape of yet to be synthesized compositions that contain O, S, and Se. The set of new compounds evaluated here are K2TiSe3, K2ZrS3, K2ZrSe3, Rb2ZrO3, Rb2ZrS3, Rb2ZrSe3, and Cs2ZrS3. We find that for these compounds the ground-state structures can be described as either antipolar (combinations with A = K or Rb) or nonpolar (combinations with A = Cs), in agreement with the conclusions of Ref. [56].
Table 1 contains the DFT-computed ground-state space group, complete with relaxed lattice parameters and volume. Next is the PBE-GGA computed band gap (Egap), and finally the difference in energy between the ground state and metastable polar state(s) for the A2BX3 series where A = K, Rb, Cs and X = O, S, Se for select compositions with B = Ti or Zr. As X increases in size from O to S to Se, the relaxed volume of the ground-state structure increases, as do the ΔE1 values to the first metastable polar state. For the B = Zr series, the increase in ΔE1 is largest for K2ZrSe3 and smallest for Cs2ZrO3 indicating that the computed switching barrier is dependent upon periodic trends such as atomic size and electronegativity. These factors also determine the variations in computed Egap, where the values are highest for oxides (3.66–4.04) and decrease for sulfides (1.28–1.88) and selenides (1.53–1.57). We predict that the chalcogenide members of the A2BX3 family, with A = K or Rb and B = Ti or Zr will be both semiconducting and AFE, given that the computed values of ΔE1 are all below 40 meV/fu.
An important consideration when proposing new AFEs is the change in volume that results from 1st order phase transition between the antipolar ground state and polar metastable stable. Reported in Table 1 are the change in volume relative to the ground state and also the % change. To compare to known AFE oxides, the largest absolute value of ΔV/V for DFT-computed metastable polar phases of PbZrO3 is 1.3% [3] and similar values are obtained for ZrO2 [48].
Additional criteria in assessing the formability of new structures, and their dynamic stability, is to investigate quantities such as their formation enthalpy and vibrational spectra. These are both presented in the supplemental materials, where the DFT GGA formation enthalpy with respect to elements in their standard state is given in Sect. 1 of the supplemental materials. Section 2 of the supplemental materials contains a representative phonon band structure, of the new compound K2ZrS3, while the phonon modes at Γ of the antipolar ground state and metastable polar state of new AFE compounds K2ZrS3, Rb2ZrO3, and Rb2ZrS3 are detailed in Sect. 3 of the supplemental materials. We find that for the 3 new AFE compounds there are no unstable vibrational modes at Γ in either the antipolar or polar states, and that all 3 have formation enthalpies below ~ − 9 eV/f.u. (or − 1.5 eV/atom), increasing their likelihood as candidate targets for synthesis.
Our overall assessment is that the compounds K2TiSe3, K2ZrS3, K2ZrSe3, Rb2ZrO3, Rb2ZrS3, and Rb2ZrSe3 are 6 new functional materials that are potentially AFE, joining known members (K2TiO3, K2ZrO3, etc.) of the family previously suggested to also be AFEs. Figure 4 is a pictorial representation of how changing either the size of the A-site (top) or X-site affects ΔE1 (green) and ΔE2 (purple). DFT calculations demonstrate that increasing the size of the A-site from K to Rb to Cs leads to a decrease in ΔE1 and ΔE2 for A2ZrO3 compounds, with Cs2ZrO3 showing no (meta)stable polar or antipolar structures. This suggests that further compositional tuning of A2ZrO3 materials, to adjust ΔE1 and ΔE2 values, should focus on compositions with mixed K and Rb. Another route to investigate how compositional tuning could affect the energy barriers of phase transitions would be to compare the changes in ΔE1 and ΔE2 values as the X anion is changed in the K2ZrX3 series. Our DFT results indicate that increasing the size of X from to O to S to Se tends to increase ΔE1 and decrease ΔE2, though these differences are still all below 40 meV/f.u.
For compositions with B = Zr, increasing the size of the A-site (a, top) in the oxides series decreases ΔE1 (green values, given in meV) and ΔE2 (purple values, given in meV). For combinations with A = K and B = Zr, increasing the size of the anion X (b, bottom) increases ΔE1, while ΔE2 values remain relatively the same.
One benefit of creating new chalcogenide or mixed chalcogenide systems using this data as a starting point is that most combinations are ensured to have decreased Egap values when compared to their pure oxide analogs. Table 1 highlights that the primary advantage of proposing new compositions such as K2ZrS3 and Rb2ZrSe3 (or mixed variants) is that they display a decreased Egap when compared to their oxide counterparts, in line with the perovskite and Ruddlesden-Popper compositions presented in Ref. [70]. Moreover, Fig. 4 shows that for specific compositions additional polar phases are potentially metastable. We find for compositions with X = Se (K2ZrSe3, Rb2ZrSe3) a polar monoclinic phase Cc is present in the energy landscape, in agreement with prior DFT investigation on Cs2ZrSe3 [56], and contains multiple polar modes, reminiscent of the conditions required for some types of hybrid improper FEs.
Compositional tuning to adjust energy barriers
The prior two subsections focused on methodology to map out the potential energy landscape of pure A2BX3 compounds, and then using this methodology to discover new compounds in the family that are as yet to be reported. Here we focus on an abbreviated approach to understanding how mixed cation systems could behave if synthesized. We are unaware of any prior studies of compositional tuning for any antipolar members of the A2BX3 family and are using this opportunity to probe if a response in ΔE1 and P will even occur with changes in A and B-site composition and ordering. Therefore, we limit ourselves to two types of cation ordering for each example. For this analysis, two known compounds of the A2BX3 family are used as end members in multiple compositional tuning schemes: K2PbO3 and Rb2PbO3. We map out the energetics of compositional tuning to assess how ΔE1 and ΔE2 change as a function of fractional cation replacement. Our first example contains mixed B-sites (1:1 mix of Sn/Pb) and our second example contains mixed A-sites (1:1 mix of Na/K and K/Rb). To create our multiple configurations, we substituted half of the cation sites of the 4 major structure types of the A2BX3 family, found in crystallographic space groups Cmcm, Pnma, Cmc21, and C2/c [56] and let them fully relax. Unlike the examples in the previous subsections, we do not create any additional polar displacements in these mixed cation systems. This is because our findings in Results & Discussion sections (a) & (b) indicated that polarization along the z-direction, found in space groups Cmc21 or its subgroup Cc were the only (meta)stable polar structural variants. All DFT-computed formation enthalpies of the series are presented in Sect. 1 of the Supplemental Materials.
In the first example we investigate 2 configurations, where Configuration 1 has the same B-sites within 1 horizontal chain of edge-sharing BX5 square pyramids and Configuration 2 has alternating B-sites within each horizontal chain. The resulting structures of Rb2(Sn1/2Pb1/2)O3 are depicted in Fig. 5, along with their fully relaxed crystallographic space groups. Substituting 1/2 of the Pb with Sn to create horizontal chain of edge-sharing BX5 square pyramids where the B-sites are the same, Configuration 1 in Fig. 5 transforms the crystallographic space groups of A2BX3 in the following ways: Cmcm ⟶ Pmma, Pnma ⟶ Pca21, Cmc21 ⟶ Pc, and C2/c ⟶ P2/c. It is important to note here that while some space groups in the series could allow for a polarization to be present (Pca21, Amm2, etc.), our DFT calculations show that these contributions are either zero or orders of magnitude smaller when compared to the Pc or Cm examples. Berry phase polarization values for all 3 Cartesian directions for each structure are reported in Table 2.
Shown on the left-hand side is a side view of each Rb2(Sn1/2Pb1/2)O3 configuration, and then to the right are each of the 4 structures and their resulting crystallographic space groups. The Rb atoms are depicted as yellow spheres, and the SnO5 and PbO5 square pyramids are light and dark blue, respectively. In this series, the structures can be thought of as generally nonpolar, antipolar, polar, and nonpolar going left to right, though there are some examples where a small nonzero polarization is present in the Cartesian y-direction due to cation ordering effects. Polarization values for each structure are tabulated in Table 2.
The ground state of Configuration 1 is the structure classified in space group Pca21, with antipolar BX5 displacement patterns, and the lowest energy metastable state is the structure classified in space group Pc, with polar BX5 displacement patterns. This metastable structure is 3 meV/fu higher in total energy and has Pz = 0.120 C/m2, an order of magnitude larger than observed for the Pca21 structure with antipolar tilts. The volume change in going from the ground state to the first metastable state is 0.51 Ang.3 or 0.11%, in line with the values reported previously in Table 1.
Substituting 1/2 of the Pb with Sn to create horizontal chain of edge-sharing BX5 square pyramids where the B-sites alternate, Configuration 2 in Fig. 5, transforms the crystallographic space groups of A2BX3 in the following ways: Cmcm ⟶ Amm2, Pnma ⟶ Pmn21, Cmc21 ⟶ Cm, and C2/c ⟶ C2. The ground state of Configuration 2 is the structure classified in space group Pmn21, with antipolar BX5 displacement patterns, and the lowest energy metastable state is the structure classified in space group Cm, with polar BX5 displacement patterns. This metastable structure is also 3 meV/fu higher in total energy and has Pz = 0.124 C/m2. The volume change in going from the ground state to the first metastable state is 28.36 Ang.3 or 6.00%, much larger than observed in Configuration 1 and the compounds reported in Table 1. Of note here is that all 4 of the structures in Configuration 2 have a small nonzero P along the y-direction, but this is an order of magnitude smaller than the Pz of the Pc structure. Switching between these 2 lowest energy structures should result in a large change in Pz. In this first example we find that B-site substitutions lead to a range of structures where ∆E values are within 30 meV/f.u., comparable to the range of values for known oxides presented in Ref. [3] (strained PbZrO3) and [48] (ZrO2).
In the second example we investigate 2 configurations, where 1/2 of the A-sites in K2PbO3 are substituted with either Na or Rb. Configuration 1 has its A-sites ordered in the vertical direction to create alternating pillars of A-sites and Configuration 2 has its A-sites ordered in the horizontal direction to create alternating rows of A-sites. The resultant configurations of (K,Na)PbO3 and (K,Rb)PbO3 are illustrated in Fig. 6, along with their fully relaxed crystallographic space groups.
Depicted on the left-hand side is a side view of each (A,K)PbO3 configuration, and then to the right are each of the 4 structures and their resulting crystallographic space groups. K is depicted as green sphere, dopant A-sites as yellow spheres, and the PbO5 square pyramids as dark blue. In this series, the structures can be thought of generally as nonpolar, antipolar, polar, and nonpolar, though there are some examples where a small nonzero polarization is present in the x direction due to cation ordering effects. Polarization values for each structure are tabulated in Table 3.
Much like the previous Rb2(Sn1/2Pb1/2)O3 example the structures exhibit a small nonzero P, though here it is in the x direction and not y, and it is smaller by almost an order of magnitude when compared to the values in Table 3. Of note here is that in each configuration only 1 structure has a sizable P comparable to BaTiO3, and that the ∆E to access this state is 3 meV for the 2 K/Rb test cases presented. Surprisingly, we find that adding Na to K2PbO3 changes the preferred ground state to one with polar displacements, and that this was the case for both Configuration 1 and 2. Configurations 1 and 2 have ground states with P = 0.198 and 0.225 C/m2, respectively, as shown in Table 3. This did not happen with (K,Rb)PbO3, where the ground state maintained its antipolar PbO5 tilt system.
Compositional tuning of K2PbO3 can change the preferred ground-state structure and adjust the switching barriers ∆E1 and ∆E2, and this is illustrated in Fig. 7. Adding a larger cation, such as Rb, to K2PbO3 does not change the computed barrier to switch from an antipolar to polar state (it stays 3 meV), but it does decrease the barrier to switch between the polar to nonpolar structures. This is one route to reduce the energy of a FE phase transition. Adding a smaller cation, such as Na, to K2PbO3 changes the nature of the ground state and increases the computed barrier to switch between them. In our example the difference to switch between polar and antipolar states increases from 3 to either 8 or 23 meV, depending upon the ordering of the A-sites. It also increases the difference in energy between the polar and nonpolar structures, from 127 to 357 or 245 meV, depending upon the ordering of the A-sites. While our studies here limited to a 50:50 mix, we can interpolate from Fig. 7 that compositions with 0 < x < 0.5 can be further investigated through experiment to determine the range of x that leads to a FE ground state. Moreover, this analysis of multiple configurations for different mixed compositions can serve as a template for future high-throughput studies of more complex compositional tuning schemes.
Conclusions
In this manuscript, we use DFT methods to expand the number of functional materials with general formula A2BX3 by developing a series of new compounds that are related to known materials. The known materials (K2PbO3, Rb2PbO3, K2TiS3, Cs2ZrSe3, etc.) are oxides, sulfides, and selenides that have already been synthesized and characterized using a variety of methods that include molten hydroxide fluxes [62], high-temperature ampule reactions [61], solid-state calcination [74], hydrothermal growth [75], and sol–gel reactions [73], and whose functional properties as FE and AFE materials has previously gone overlooked. The main goals of this study are to (a) develop methodology for future high-throughput studies, (b) more fully map out the potential energy landscape of antipolar and polar A2BX3 compositions, (c) predict new semiconductive AFE and FE materials that are yet to be synthesized, and (d) investigate the effects of compositional tuning on known structures.
We developed a symmetry-based procedure amenable to high-throughput investigations to search for (meta)stable polar displacements and then showed that for a test set of known oxides, only a few polar displacement patterns are (meta)stable. These polar displacements are then used to map out the potential energy landscape of compositions that are as yet to be synthesized. This led to 6 new example AFEs, where select member such as K2ZrS3 and Rb2ZrSe3 demonstrate a reduced Egap compared to their oxide analogs while maintaining the same relative ground-state and metastable structures. Our analyses indicate that these new example AFEs, as well as the known materials on which they are based, are prime targets for DFT calculations that should include (i) nudged elastic band pathways to evaluate multiple switching pathways and their associated barriers, (ii) complete phonon spectra for all ground and metastable state structures, and (iii) PBE0- or GW-type calculations to compute Egap values beyond the semi-local GGA values reported here in our preliminary assessments.
Once these materials are synthesized, structurally characterized, and their functional properties are mapped out, they could also be used as end members in compositional tuning schemes. The compositional tuning schemes presented here can be used as a guide for experiment: we predict that adding Na to K2PbO3 changes the preferred ground state from antipolar to polar, while adding Rb to K2PbO3 can decrease the barrier for FE switching of metastable states. Much like the new chalcogenides listed in the previous paragraph, these mixed oxides could serve as templates for phonon calculations to assess how composition affects vibrational frequency, in line with landmark studies on perovskite oxides that explored how to understand and control the soft modes that lead to FE instabilities.
Future studies using previously synthesized Pb-free compounds not explored here (K2SnO3, K2ZrO3, K2TiS3, etc.) could also expand upon these points and examine further the optimized properties that could result from mixing both A- and B-sites. Delving further, this would result in designed materials with more than 1 dominant metastable polar state with adjustable ∆E1 and ∆E2 values and open new lines of research into applications that could employ hybrid improper FEs with multiple tunable switching barriers. In total, this work has resulted in new examples of FE and AFE oxides, sulfides, and selenides, some of which have a Egap amenable to photovoltaic applications, while others appear to be more useful as solid-state materials for energy storage.
Materials and methods
The ground-state calculations presented here employ periodic DFT methods [76, 77] and are carried out using ABINIT, an open-source software package [78, 79]. All atoms are represented using optimized norm-conserving Vanderbilt (ONCV) pseudo-potentials [80,81,82] and a plane-wave cutoff of 50 Ry. Bulk structural relaxations use a 6 × 6 × 6 k-point grid [83] and the energy convergence criteria for self-consistent relaxations was 5 × 10–6 eV. This stringent criteria allows for ∆E comparisons down to atleast 1 meV and also results in relaxed structures with maximum residual forces of 1–5 meV/Å per atom per Cartesian direction. Wherever applicable, we report polarization using the Berry phase approach [84, 85]. All calculations are performed at the GGA level using the Wu-Cohen (WC) modified PBE-GGA exchange correlation function for solids [86, 87]. We choose the WC-modified PBE-GGA because of its (i) improved agreement with experimental structures for a wide range of semiconductors, including oxides and chalcogenides [88], (ii) ability to match well the properties of known FEs PbTiO3 and BaTiO3 [87], and (iii) utility in investigating phase transitions of FEs [89], even when compared to meta-GGA functionals [90].
The DFT-computed formation enthalpy presented in Sect. 1 of the Supplemental Materials employ crystal structures of the ground-state atoms and molecules, calculated using known crystal structures of Na, K, Rb, Ti, Zr, Cs, S, and Se that were found in the ICSD, consistent with the methods outlined in Ref [91]. For a cursory evaluation of the dynamic stability of the new compounds Rb2ZrO3, K2ZrS3, and Rb2ZrS3, we use ABINIT to compute the vibration modes of the antipolar ground state (Pnma) and the first metastable polar state (Cmc21). These calculations are performed at the Γ point using a 6 × 6x6 k grid using DFPT methods Refs. [92, 93] for these 24 atom cells. The vibrational modes are reported in Sect. 3 of the Supplemental Materials and are accompanied by the mode effective charges and contributions to the dielectric constant, as detailed in Refs. [94, 95]. A representative phonon band structure for the new compound K2ZrS3 is presented in Sect. 2 of the Supplemental Materials.
To more fully map out the potential energy landscape of the A2BX3 family of materials, we employ a symmetry-guided approach using the ISOTROPY software suite [96]. Our first step is to create polar displacements in both the high symmetry nonpolar parent structure (in space group Cmcm) and antipolar subgroup structure (in space group Pnma). These displacement patterns are created by adding 0.02 to 0.05 reduced coordinates to the cation coordinates and subtracting 0.02 to 0.05 reduced coordinates from the anion coordinates, in one or more Cartesian directions. The polar displacements are also accompanied by an increase in 0.4 to 0.8 Ang. of the lattice constant in that direction, depending upon the size of the cation A-sites (e.g., rRb > rK) and anion X-sites (e.g., rSe > rS > rO). The set of structures with polar displacements are then used as the input for our DFT calculations, where we employ FINDSYM [97, 98] to obtain the crystallographic space group, and then use ISODISTORT [99, 100] to obtain the sets of irreducible representations (irreps) that describe the induced polar displacements before the structures are let to fully relax. The polar irreps from this pre-relaxation analysis are depicted in Fig. 2.
Data availability
Data will be made available on reasonable request.
References
J.W. Bennett, K.F. Garrity, K.M. Rabe, D. Vanderbilt, Phys. Rev. Lett. 109, 167602 (2012)
J.W. Bennett, K.F. Garrity, K.M. Rabe, D. Vanderbilt, Phys. Rev. Lett. 110, 017603 (2013)
S.E. Reyes-Lillo, K.M. Rabe, Phys. Rev. B. 88, 180102 (2013)
N.A. Benedek, C.J. Fennie, J. Phys. Chem. 117, 13339 (2013)
N.A. Benedek, T. Birol, J. Mater. Chem. C 4, 4000 (2016)
R. Zhang, B.M. Abbett, G. Read, F. Lang, T. Lancaster, T.T. Tran, P.S. Halasyamani, S.J. Blundell, N.A. Benedek, M.A. Hayward, Inorg. Chem. 55, 8951 (2016)
B. Monserrat, J.W. Bennett, K.M. Rabe, D. Vanderbilt, Phys. Rev. Lett. 119, 036802 (2017)
T. Zhu, G. Khalsa, D.M. Havas, A.S. Gibbs, W. Zhang, P.S. Halasyamani, N.A. Benedek, M.A. Hayward, Chem. Mater. 30, 8915 (2018)
J. Young, J.M. Rondinelli, Phys. Rev. Mater. 2, 065406 (2018)
Z. Fei, W. Zhao, T.A. Palomaki, B. Sun, M.K. Miller, Z. Zhao, J. Yan, X. Xu, D.H. Cobden, Nature 560, 336 (2018)
J.W. Bennett, K.M. Rabe, J. Solid State Chem. 195, 21 (2012)
J.W. Bennett, J. Solid State Chem. 281, 121045 (2020)
K.M. Rabe, Antiferroelectricity in Oxides: A Reexamination (Wiley, New York, 2013)
I. Grinberg, D.V. West, M. Torres, G. Gou, D.M. Stein, L. Wu, G. Chen, E.M. Gallo, A.R. Akbashev, P.K. Davies, J.E. Spanier, A.M. Rappe, Nature 503, 509 (2013)
K.T. Butler, J.M. Frost, A. Walsh, Energy Environ. Sci. 8, 838 (2015)
Z. Xiao, Y. Yuan, Y. Shao, Q. Wang, Q. Dong, C. Bi, P. Sharma, A. Gruverman, J. Huang, Nat. Mater. 14, 193 (2015)
S.E. Young, J.Y. Zhang, W. Hong, X. Tan, J. Appl. Phys. 113, 054101 (2013)
C.W. Ahn, G. Amarsanaa, S.S. Won, S.A. Chae, D.S. Lee, I.W. Kim, ACS Appl. Mater. Interfaces 7, 26381 (2015)
M. Zhou, R. Liang, Z. Zhou, S. Yan, X. Dong, ACS Sustain. Chem. Eng. 6, 12755 (2018)
L. Yang, X. Kong, F. Li, H. Hao, Z. Cheng, H. Liu, J.F. Li, S. Zhang, Progr. Mater. Sci. 102, 72 (2019)
R.E. Cohen, Nature 358, 136 (1992)
N. Sicron, B. Ravel, Y. Yacoby, E.A. Stern, F. Dogan, J.J. Rehr, Phys. Rev. B 50(18), 13168 (1994)
J. Wang, J.B. Neaton, H. Zheng, V. Nagarajan, S.B. Ogale, B. Liu, D. Viehland, V. Vaithyanathan, D.G. Schlom, U.V. Waghmare, N.A. Spaldin, K.M. Rabe, M. Wuttig, R. Ramesh, Science 299, 1719 (2003)
J.M. Rondinelli, N.A. Spaldin, Adv. Mater. 23, 3363 (2011)
J.W. Bennett, I. Grinberg, A.M. Rappe, J. Am. Chem. Soc. 130, 17409 (2008)
K. Takae, H. Tanaka, P. N. A. S. 115, 9917 (2019)
P.F. Li, W.Q. Liao, Y.Y. Tang, H.Y. Ye, Y. Zhang, R.G. Xiong, J. Am. Chem. Soc. 139, 8752 (2017)
N.A. Benedek, C.J. Fennie, Phys. Rev. Lett. 106, 107204 (2011)
S. Yoshida, K. Fujita, H. Akamatsu, O. Hernandez, A.S. Gupta, F.G. Brown, H. Padmanabhan, A.S. Gibbs, T. Kuge, R. Tsuji, S. Murai, J.M. Rondinelli, V. Gopalan, K. Tanaka, Adv. Funct. Mater. 28, 1801856 (2018)
H. Djani, E.E. McCabe, W. Zhang, P.S. Halasyamani, A. Feteira, J. Bieder, E. Bousquet, P. Ghosez, Phys. Rev. B. 101, 134113 (2020)
N.A. Benedek, J.M. Rondinelli, N. Djani, P. Ghosez, P. Lightfoot, Dalton Trans. 44, 10543 (2015)
P. Boullay, J. Tellier, D. Mercurio, M. Manier, F.J. Zuniga, J.M. Perez-Mato, Solid State Sci. 14, 1367 (2012)
J.B. Li, Y.P. Huang, G.H. Rao, G.Y. Liu, J. Luo, J.R. Chen, J.K. Liang, Appl. Phys. Lett. 96, 222903 (2010)
A. Snedden, C.H. Hervoches, P. Lightfoot, Phys. Rev. B. 67, 092102 (2003)
M. Clin, J.P. Rivera, H. Schmid, Ferroelectrics 108, 207 (1990)
H. Schmid, Ferroelectrics 162, 317 (1994)
B.J. Kooi, B. Noheda, Science 353, 221 (2016)
W. Wan, C. Liu, W. Xiao, Y. Yao, Appl. Phys. Lett. 111, 132904 (2017)
M.S. Haque, J.R. Hardy, Phys. Rev. B. 21, 245 (1980)
J.M. Perez-Mato, F. Gaztelua, G. Madariaga, M.J. Tello, J. Phys. C 19, 1923 (1986)
E. Sawaguchi, H. Maniwa, S. Hoshino, Phys. Rev. 83, 1078 (1951)
D.J. Singh, Phys. Rev. B 52, 12559 (1995)
L. Zhao, Q. Liu, J. Gao, S. Zhang, J.F. Li, Adv. Mater. 29, 1701824 (2017)
Z. Liu, T. Lu, J. Ye, G. Wang, X. Dong, R. Withers, Y. Liu, Adv. Mater. Technol. 3, 1800111 (2018)
S. Han, X. Liu, Y. Liu, Z. Xu, Y. Li, M. Hong, J. Luo, Z. Sun, J. Am. Chem. Soc. 141, 12470 (2019)
Z. Wu, X. Liu, C. Ji, L. Li, S. Wang, Y. Peng, K. Tao, Z. Sun, M. Hong, J. Luo, J. Am. Chem. Soc. 141, 3812 (2019)
T. Wakamatsu, G. Kawamura, T. Abe, Y. Nakahira, S. Kawaguchi, C. Moriyoshi, Y. Kuroiwa, I. Terasaki, H. Taniguchi, Inorg. Chem. 58, 15410 (2019)
S.E. Reyes-Lillo, K.F. Garrity, K.M. Rabe, Phys. Rev. B. 90, 140103 (2014)
M.H. Park, Y.H. Lee, H.J. Kim, Y.J. Kim, T. Moon, K.D. Kim, J. Muller, A. Kersch, U. Schroeder, T. Mikolajick, C.S. Hwang, Adv. Mater. 27, 1811 (2015)
A. Girard, H. Taniguchi, S.M. Souliou, M. Stekiel, W. Morgenroth, A. Minelli, A. Kuwabara, A. Bosak, B. Winkler, Phys. Rev. B. 98, 134102 (2018)
H. Taniguchi, A. Kuwabara, J. Kim, Y. Kim, H. Moriwake, S. Kim, T. Hoshiyama et al., Angew. Chem. Int. Ed. 52, 8088 (2013)
T. Murata, H. Akamatsu, D. Hirai, F. Oba, S. Hirose, Phys. Rev. Mater. 4, 104419 (2020)
S.T. Zhang, A.B. Kounga, W. Jo, C. Jamin, K. Seifert, T. Granzow, J. Rodel, D. Damjanovic, Adv. Mater. 21, 4716 (2009)
C. Kittel, Phys. Rev. 82, 729 (1951)
P. Toledano, M. Guennou, Phys. Rev. B. 94, 014107 (2016)
J.W. Bennett, J. Phys. Chem. C. 124, 19413 (2020)
R. Hoppe, H.J. Roehrborn, H. Walker, Naturwissenschaften 51, 86 (1964)
R. Hoppe, H.D. Stover, Zeitschrift fuer Anorg. und Allgemeine Chem. 437, 123 (1977)
H. Stoll, R. Hoppe, Zeitschrift fuer Anorg. und Allgemeine Chem. 551, 151 (1987)
P. Panek, R. Hoppe, Zeitschrift fuer Anorg. und Allgemeine Chem. 393, 13 (1972)
K.O. Klepp, A. Kolb, Z. Kristallogr. 213, 694 (1998)
H.S. Khalsa, M.D. Smith, H.C. zur Loye, Mater. Res. Bull. 44, 91 (2009)
M.R. Harrison, M.G. Francesconi, Coord. Chem. Rev. 255, 451 (2011)
K.F. Garrity, Phys. Rev. B. 97, 024115 (2018)
N.V. Ter-Oganessian, V.P. Sakhnenko, J. Phys. 32, 275401 (2020)
J.W. Bennett, I. Grinberg, A.M. Rappe, Phys. Rev. B. 79, 235115 (2009)
M. Ong, D.M. Guzman, Q. Campbell, I. Dabo, R.A. Jishi, J. Appl. Phys. 125, 235702 (2019)
J.A. Brehm, J.W. Bennett, M.R. Schoenberg, I. Grinberg, A.M. Rappe, J. Chem. Phys. 140, 224703 (2014)
J.A. Brehm, H. Takenaka, C.W. Lee, I. Grinberg, J.W. Bennett, M.R. Schoenberg, A.M. Rappe, Phys. Rev. B. 89, 195202 (2014)
R. Jaramillo, J. Ravichandran, APL Mater. 7, 100902 (2019)
K.O. Klepp, Z. Naturforsch 47b, 201 (1992)
B.M. Gatehouse, D.J. Lloyd, J. Solid State Chem. 2, 410 (1970)
R. Mishra, M. Ali, S.R. Bharadwaj, D. Das, J. Therm. Anal. Calorim. 66, 779 (2001)
Y. Masubuchi, R. Miyazaki, H. Kirkuchi, T. Motohashi, S. Kikkawa, Dalton Trans. 43, 13751 (2014)
R.J. Terry, C.D. McMillen, X. Chen, Y. Wen, L. Zhu, G. Chumanov, J.W. Kolis, J. Cryst. Growth 493, 58 (2018)
P. Hohenberg, W. Kohn, Phys. Rev. 136, B864 (1964)
W. Kohn, L.J. Sham, Phys. Rev. 140, A1133 (1965)
X. Gonze, B. Amadon, P.M. Anglade, J.M. Beuken, F. Bottin, P. Boulanger, F. Bruneval, D. Caliste, R. Caracas, M. Cote et al., Comput. Phys. Commun. 180, 2582 (2009)
X. Gonze, F. Jollet, F. Abreu-Araujo, D. Adams, B. Amadon, T. Applencourt, C. Audouze, J.M. Beuken, J. Bieder, A. Bokhanchuk, E. Bousquet et al., Comput. Phys. Commun. 205, 106 (2016)
D.R. Hamann, M. Schluter, C. Chiang, Phys. Rev. Lett. 43, 1494 (1979)
D. Vanderbilt, Phys. Rev. B 41, 7892 (1990)
D.R. Hamann, Phys. Rev. B. 88, 085117 (2013)
H.J. Monkhorst, J.D. Pack, Phys. Rev. B 13, 5188 (1976)
R.D. King-Smith, D. Vanderbilt, Phys. Rev. B 47, 1651 (1993)
R. Resta, Rev. Mod. Phys. 66, 899 (1994)
J.P. Perdew, K. Burke, M. Ernzerhof, Phys. Rev. Lett. 77, 3865 (1996)
Z. Wu, R.E. Cohen, Phys. Rev. B. 73, 235116 (2006)
F. Tran, R. Laskowski, P. Blaha, K. Schwarz, Phys. Rev. B. 75, 115131 (2007)
P. Ganesh, R.E. Cohen, J. Phys. 21, 064225 (2009)
A. Paul, J. Sun, J.P. Perdew, U.V. Waghmare, Phys. Rev. B. 95, 054111 (2017)
J.W. Bennett, D.T. Jones, B.G. Hudson, J. Melendez-Rivera, R.J. Hamers, S.E. Mason, Environ. Sci. Nano 7, 1642 (2020)
X. Gonze, C. Lee, Phys. Rev. B. 55, 10355 (1997)
X. Gonze, Phys. Rev. A. 52, 1096 (1995)
E. Cockayne, J. Eur. Ceram. Soc. 23, 2375 (2003)
J.W. Bennett, I. Grinberg, A.M. Rappe, Phys. Rev. B. 73, 180102R (2006)
H.T. Stokes, D.M. Hatch, B.J. Campbell, http://iso.byu.edu
H.T. Stokes, D.M. Hatch, Phys. Rev. B. 30, 4962 (1984)
H.T. Stokes, D.M. Hatch, J. Appl. Cryst. 38, 237 (2005)
B.J. Campbell, H.T. Stokes, D.E. Tanner, D.M. Hatch, J. Appl. Cryst. 39, 607 (2006)
S. Kerman, B.J. Campbell, K.K. Satyavarapu, H.T. Stokes, F. Perselli, J.S.O. Evans, Acta Cryst. A68, 222 (2012)
J. Towns, T. Cockerill, M. Dahan, I. Foster, K. Gaither, A. Grimshaw, V. Hazlewood, S. Lanthrop, D. Lifka, G.D. Peterson, R. Roskies, J.R. Scott, N. Wilkins-Diehr, Comput. Sci. Eng. 16, 62 (2014)
Acknowledgments
JWB acknowledges resources provided by the College of Natural and Mathematical Sciences and the Department of Chemistry and Biochemistry at the University of Maryland, Baltimore County. This work used the Extreme Science and Engineering Discovery Environment (XSEDE, [101]), which is supported by National Science Foundation Grant number ACI-1548562 through start up allocation ID TG-CHE190075 and renewal allocation TG-CHE200108. The hardware used in some of the computational studies is part of the UMBC High-Performance Computing Facility (HPCF). The facility is supported by the US National Science Foundation through the MRI program (Grant nos. CNS-0821258, CNS-1228778, and OAC-1726023) and the SCREMS program (Grant no. DMS-0821311), with additional substantial support from the University of Maryland, Baltimore County (UMBC).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Khan, A.C., Cook, A.S., Leginze, J.A. et al. Developing new antiferroelectric and ferroelectric oxides and chalcogenides within the A2BX3 family. Journal of Materials Research 37, 346–359 (2022). https://doi.org/10.1557/s43578-021-00410-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/s43578-021-00410-3