Abstract
The transport properties of molecular wire comprising of B40 fullerene are investigated by employing density functional theory (DFT) and non-equilibrium green’s function (NEGF) methodology. The quantum transport is evaluated by calculating the density of states, transmission spectra at various bias voltages, molecular energy spectra, HOMO-LUMO gap, current–voltage curve, and transmission pathways. In context to its properties, results show that by increasing the length of molecular wire, the device exhibits rectification ratio and prominent NDR behavior. I–V curve scrutinizes that as the length of wire is increased the curve becomes non-linear. This non-linear behavior is more prominent in the case when the length of wire is increased up to six fullerene cages significant rectification ratio (R.R) and negative differential resistance (NDR) comes into the picture. The excellent negative differential resistance ensures that a device with at least six molecular wires can be used as a tunnel diode.
Graphic abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The last two decades have brought a revolution in the field of nanomaterials; researchers and engineers have been actively engaged in finding various applications of nanomaterials in the field of medical, environment, and electronics. Ever since the famous lecture “There is plenty of room at the bottom” by Professor R. Feynman, the field of nanotechnology has gained great impetus [1]. Due to the availability of organic and inorganic material, the field of molecular electronics has become the center of attention for the research community. Researcher’s started the research in the field of carbon fullerenes and their transport behavior under equilibrium and non-equilibrium conditions. In 1985 Kroto discovered the carbon fullerene C60 which was marked as a breakthrough in the field of mole electronics [2]. Prinzbach amalgamed the C20 fullerene molecule in the year 2000 which began the research in this area [3]. Researchers altered the carbon fullerene properties by decorating the fullerene cage with a different atom or by endohedral or exohedral placement. Later on, various properties of C20 were scrutinized utilizing the local density approximation approach [4].
Fullerenes have intrigued wide interest due to their functional properties and various applications in molecular electronics [5]. The synthesis of B40 in 2014 brought the revolutionizing change in the field of molecular electronics. The borospherene (B40) is a highly stable fullerene, that comprises two hexagons and four heptagons [5] and comprises of D2d symmetry arrangement corresponding to the Chinese red lantern [6]. Borospherene as a material has shown its ability in various device applications. He and Zeng inquired about the spectral properties of B40 and deduced that it has more appropriate spectral properties in contrast to C60 [5]. Later on, Yang et. al investigated B40 molecular junction which exhibits excellent optoelectronic properties with a large rectification ratio and NDR characteristics [7]. Tang et. al explored that Sc@B40 is capable of absorbing hydrogen gas molecules [8]. Furthermore, research discloses that borospherene has super atomic characteristics comprising of 2s, 2p, 2d, and 2f orbitals along with 1s, 1p, 1d, and 1f orbitals [9]. The research work of Keyhanian et. al probed the B40 potential for aniline adsorption [10]. Mn and Fe atoms were doped and encapsulated for enhancing the fullerene properties. B40 emerged as an excellent candidate in molecular electronics whose properties can be modified to a large extent by adding or removing elements. Thus, Kaur et. al designed B40 molecular junction by doping Al, Si, and S from the periodic table [11]. Shakerzadeh et. al scrutinize electronic and non-linear optical properties (NLO) of M@B40 (M = first row transition metals of the periodic table) and results showcased that boron-based devices can be used to design electro-optical materials. The molecular junction comprising B40 exhibits a high rectification ratio and NDR [12]. Due to the large surface area and presence of both acidic and basic sites, B40 has been extensively utilized as a sensor for the detection of various toxic gases like CO2, ammonia, phosgene gas, etc. [13, 14]. Borospherene (B40) has shown its application as a nanocatalyst for hydrogen splitting [15]. Hence transport properties of B40 can be altered either by decorating borospherene with transition metals or by dopping or encapsulation of different atoms from the periodic table. Maniei et. al doped lithium in Borospherene and formed a device that can detect nitrogen dioxide, thus giving a sensing application [16]. Cheng et. al utilizing DFT formalism scrutinized guanine nucleo-base of DNA with B40. From the results, it was concluded that B40 can be used for sensing guanine nucleo-base due to its electrical conductivity [17]. Zhang et. al probed yttrium-doped boron fullerene for hydrogen storing capacity [18]. It was inferred that one Y atom can capture five hydrogen molecules. Kosar et. al studied B40 fullerene as an energy storage material. They probed the interaction of B40 fullerene with Na and Na+ [19]. In July 2020, Kaur et. al probed the potential of B40 to recognize radium and radon in water [20]. Wang et. al in 2017 designed a molecular wire with B40 to study the interaction between B40 fullerene [21]. It was inferred that a reduction in the bandgap from 3.18 to 1.10 eV was noticed as an increase in B40 fullerene. Although they have studied the interaction properties of B40 as a molecular wire, a junction using gold electrodes has not been studied yet.
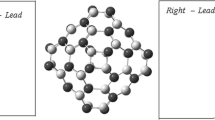
In the present work, a B40-based molecular wire (Fig. 1) was designed intending to study the change in electronic transport properties both at equilibrium and non-equilibrium by increasing the length of the wire. While designing the molecular junction linkers are not used as pure B40 molecular junction has shown excellent electronic properties without linker’s and when linkers have used the conductance of the device is considerably decreased [22]. A comparative study of six different junctions has been investigated. DFT-NEGF methodology is utilized to calculate transmission spectra, molecular energy levels, I–V curve, HLG, and transmission pathways of all the molecular junctions under consideration.
Results and discussion
Quantum transport at equilibrium
The quantum transport was investigated under equilibrium conditions, i.e., 0 V. Density of states (DOS) was determined for all B40 devices to understand thoroughly the transport properties. DOS gives knowledge about the quantum states that are vigorously involved in the transmission. Figure 2 shows a comparative graph for six B40 devices, where peaks are anticipated on both sides of the fermi level (EF). From Fig. 2, it is evident that for the 5th device and 6th device the peaks are more prominent below the Fermi level (EF); hence the highest occupied molecular orbital (HOMO) dominates the transmission for both devices, whereas for other devices peaks are more prominent above the EF; hence the lowest unoccupied molecular orbital (LUMO) dominates the transmission in the other four devices. The higher the value of D(E) for peaks, the greater is the involvement of that particular state in transmission and hence greater conduction [23].
For all the devices at 0 V, the molecular energy spectrum was studied. The HOMO, LUMO, and HLG in all the molecular junctions are tabulated in Table 1. From the table, it is deduced that HOMO–LUMO orbitals shift close to Fermi level, as the number of B40 cages between the electrodes is increased. This results in a decreased HOMO–LUMO gap (HLG) in the devices, as we increase the number of fullerene stages in contrast to the single B40 molecular junction which has the highest energy gap. The HOMO–LUMO gap (HLG) energies for molecular chain are calculated to be D1 (0.563 eV) > D2 (0.513 eV) > D3 (0.436 eV) > D4 (0.324 eV) > D5 (0.055 eV) > D6 (0.054 eV), respectively, where D1, D2, D3, D4, D5, and D6 represent the different molecular junctions.
The transmission spectra exploit the molecular orbital energies for applied bias, to find out at which energies electron transfer would be strongest [24]. Figure 3 shows the transmission spectra of all devices considered at zero bias. The more number of peaks in transmission spectra the higher the transmission. If the transmission peaks are wider, it signifies the stronger coupling between electrodes and central molecules, thus resulting in more transmission. On the other hand, sharper peaks signify poor coupling and hence reduced transmission. In I–V characteristics, the position of the Fermi level is considered to be an important factor, as it helps in identifying the conductive characteristics of elements [25]. In the transmission spectra at zero bias, the Fermi level is considered to be at zero EF = 0. T(E) curves of all devices were determined in the energy range of − 1 to 1 eV. Comparing all the devices, it is visualized that as we increase the length of the molecular chain, higher number of transmission peaks are noticed near the Fermi level [21].
Quantum transport under non-equilibrium conditions
After performing simulations and calculations, we first plotted the I–V curve. The current was determined at various bias voltages from − 1 to + 1 V with a step size of 0.2 V. From Fig. 4 it is visualized that current is always symmetric for all the devices. The curve is completely linear in the case of a single B40 molecular junction implying that current flows across the junction without any barrier. As the length of the wire is increased further, the curve becomes non-linear. The non-linear behavior increases with an increase in the length such that for the device having six B40 cages, significant NDR comes into the picture [21].
The negative differential resistance can be defined as the drop in electron mobility with an increase in an electric field which means that a device will exhibit a decrease in current with an increase in applied voltage. For the I–V curve of molecular chain devices, as shown in the figure, a prominent negative differential resistance (NDR) behavior is observed. By increasing molecular chain length, NDR behavior becomes more promising. From the figure, it is envisaged that for the molecular wire comprising of four B40 stages NDR is observed in the short range of − 0.62 V to 0.64 V, and for five stages NDR is observed in the voltage range of − 0.67 V to 0.69 V, and then for six stages, NDR is observed in the voltage range of − 0.75 V to 0.76 V. Thus, B40 molecular wire comprising of four, five, or six B40 stages can be effectively utilized in several electronic applications like oscillators, amplification, and digital applications [21].
The rectification ratio R can be defined as R = I(V)/I(−V). The calculated rectification ratio is shown in the figure. From Fig. 5, it is evident that molecular chain devices with six B40 fullerenes exhibit an enhanced rectification ratio and can be used as a tunnel diode. It is inferred that when 0.4 V bias is applied to a molecular chain consisting of one, two, and five B40 stages, rectification ratio is unity, whereas for the molecular wire consisting of three and six B40 stages rectification ratio is 0.93 and in the case of four B40 stages rectification ratio is observed as 0.99.
From Table 1, it is quite evident that D6 with six B40 cages has HLG less than a single B40 cage even though a single cage device has increased the flow of current. Here’s the reason why HLG is less for all other devices, even though they have lower values of current, is the weak bonding of electrons although the accumulation of charge carriers on all sides is more prominent yet the flow of current is less. Also, the free electrons are responsible for the reduction in current, as we keep on increasing the length of the molecular chain the number of free electrons decreases which further leads to a reduction in current.
To understand the quantum transport properties, the zero bias transmission spectrum is not sufficient. Therefore, the change of transport properties under various bias voltages from − 1 to 1 V is investigated. Figures 6 and 7 present a graphical representation of the various bias voltages.
From the graph of transmission spectra at − 1 V and + 1 V, it is visualized that for positive bias magnitude of the peaks is increased in contrast to negative bias. And this variation in magnitude can be seen for molecular wire comprising of three, four, five, and six borospherene cages. Also, there is a shift in the position of peaks for these devices. In the case of transmission spectra at + 1 V and at − 1 V, for the molecular wire comprising of six cages, it is visualized that magnitude increases for a positive bias in contrast to a negative bias. For the negative bias, first peak near the Fermi level is at − 0.04 eV with a magnitude of 0.965, whereas for the positive bias first peak lies at the Fermi level with a magnitude of 1.91.
In the transmission spectra for bias voltage at − 0.8 V and + 0.8 V, it is interpreted that positive bias magnitude is increased in contrast to negative bias. In the case of molecular wire comprising of six cages, it is interpreted that for negative bias first peak lies close to the Fermi level at − 0.08 eV with a magnitude of 1.55, whereas for positive bias peaks close to the Fermi level with a magnitude of 2.85. Furthermore, the transmission spectra at + 0.6 V and − 0.6 V negative bias closest peaks lie at 0.08 eV with a magnitude of 1.92, whereas for positive bias closest peaks lie at − 0.04 eV with a magnitude of 2.31 which depicts that with change in bias states participating in conduction also change. For the transmission spectra at + 0.4 V and − 0.4 V, in the case of molecular wire comprising of six fullerene cages, in the positive bias, the first peak lies at − 0.04 eV with a magnitude of 3.219, whereas for negative bias the first peak lies at 0.04 eV with a magnitude of 2.709. Thus, it is deduced that magnitude of the peak is increased for positive bias.
Next, for the transmission spectra at + 0.2 V and − 0.2 V in the case of molecular wire comprising of six cages, it is visualized that for positive bias first peak lies at 0.04 eV with a magnitude of 3.46, whereas for negative bias first peak lies at 0.04 with a magnitude of 3.004. Hence it is inferred that for positive bias values of peaks are enhanced in contrast to negative bias. So from the transmission spectra graphs, it is concluded that the shape of the curve is the same but amplitude changes with applied voltage. Moreover, by varying the bias voltages peaks don’t move much from their position. Also as the bias is increased the states that were engaged in conduction don’t change [25].
To have a clear image of how the length of the wire affects the conduction of B40 fullerene cages, we have further analyzed the transmission pathways. The transmission pathway is a quantitative means to understand the current flow between molecule and electrodes in terms of the local contribution. In transmission pathways, the local transmission components are represented by arrows that superimpose on the molecular geometry [32]. If the value of local transmission within a pair of atoms is somewhat 10% of maximum local transmission, then only an arrow is drawn. These local transmission components can either follow the single pathway or multiple pathways. The total current I(V) equals the sum of local currents within the pairs of atoms p and q, where p is on one side of the surface and q is on the other side of the surface,
Figure 8 represents the local flow of current in form of three different lines (dark, light, and dotted lines). The light lines give components of the transmission that are in direction of the net current flow; on the other hand, dark lines give components in opposite direction, thereby reducing the net current, whereas the dotted lines represent the scattering of electrons. The transmission pathways of six different B40 devices give information about current flow through bridging molecules bound to metallic electrodes. The flow of current is along the spherical surface of the cage, whereas if the length of the device is increased there is a reduction in the flow of current. In the four, five, and six cage devices, the opposite direction current flows directly through inside the cage. From Fig. 8, it can be seen that as the length of the molecular chain is increased, there is a reduction in current.
Conclusion
In this study, B40 fullerene is used to design a single molecular junction and molecular wire comprising fullerene cages. To enumerate the density of states, transmission spectra, molecular energy spectra, HLG, I–V curve, and transmission pathways the DFT along with NEGF formalism have been employed. Single molecular junction has the highest value of current, whereas on increasing the length of wire value of current keeps on reducing. This reduction in current is seen in transmission pathways where a device with six fullerenes has increased backscattered components in comparison with a single B40 molecular wire that has more forward components. When six B40 fullerenes are added between the junctions HLG gap reduces to 0.054 eV. It is deduced that for the first four devices LUMO orbitals play a dominant role in the transmission and for the other two devices HOMO orbitals dominate the transmission. The I–V curve depicts the non-linear behavior for molecular junctions as the length of wire is increased. Specifically, NDR behavior is observed in the I–V curve due to an increase in the length of the molecular wire. It means that molecular junctions comprising at least six B40 fullerene cages can be used as molecular wire. Thus excellent negative differential resistance ensures that a device with at least six molecular wires can be used as a tunnel diode.
Methodology
In this paper, necessary calculations are performed utilizing Virtual Nano Lab software which is included in Atomistix Toolkit [26]. Virtual Nano Lab (VNL) immolates various calculation methods. Density Functional Theory (DFT) was chosen due to its better accuracy in comparison to extended Huckle theory (EHT) [27]. Generalized Gradient Approximation (GGA) of Perdew-Burke-Ernzerhof is used to calculate exchange–correlation energy [27]. For all the devices, double zeta polarized basis set was used along with a density mesh cut-off energy of 150Ry. The fullerenes (B40) were sandwiched between the gold electrodes to form six different devices with \(\langle {1},{1},{1}\rangle\) electrode orientation. The single covalent bond is formed between the fullerenes and metallic leads. The minimum length of the device was 45 \(\AA\) as the length of the wire is increased the maximum length of the device is 75 \(\AA\).
To probe the use of B40 as a molecular wire, a molecular junction has been designed. B40 fullerene cages are sandwiched between the gold electrodes forming a single molecular junction and then by increasing the length of wire up to six fullerene cages other molecular junctions are formed. The device encompasses three parts, namely the left electrode, central region, and right electrode.
To calculate current characteristics, Landauer formalism is utilized at various applied voltages [28, 29]. Landauer Buttiker formalism exploits the transmission probability T(E, V):
In Eq. 1. T(E, V) signifies transmission function and \({\mu }_{\mathrm{L}}/{\mu }_{\mathrm{R}}\) represents a bias window. In this study, the transmission was determined as [27]
where \({\Gamma }_{1}\) and \({\Gamma }_{2}\) are coupling functions related to left/right electrodes, respectively. It provides information regarding the kind of contact among molecule and metallic contacts, and GM(E, V) represents Green’s function. The values for the electrochemical potential for both left/right electrodes can be written as follows:
From Eqs. 3 and 4, EF exhibits the equilibrium Fermi function, e represents the charge on the electron, and \(\eta\) gives the information on how potential difference (V) is divided among two metallic leads. When the molecule in the molecular junction has a minimal response on the conductance curve, then \(\eta\) describes the potential profile of the molecule [29, 30].
To explore the transfer of electrons in molecular junctions, transmission pathways were calculated. The Green’s function generally gives the value of current at the metal–molecule surface or within the metal [31], whereas transmission pathways describe the flow of current in electrode-molecule-electrode. The total equals the sum of local currents among all the pairs of atoms p and q, where p denotes one side of the surface and q denoted the other side of the surface.
In transmission pathways, electronic bonding is termed as “through-bond” and “through-space”. “Through-bond” is a term is used where atoms are directly connected or connected in a multiple atom sequence [32]. On the other hand, the “through-space” term is used where electronic coupling takes place between the non-linked atoms. These are weak interactions such as hydrogen bonds. Thus, in the case of a small molecule, the representation of local current will be influenced by through-bond terms. The local transmission via a molecule offers a path to understand the impression about the type of dynamics between the molecular assembly both in chemical and geometric essence and electronic bonding. The flow of current is given by local transmission components either in the single pathway or multiple pathways. By superimposing the device on the transmission pathways result, one can understand the flow of current. The direction of the current is shown by arrows colored in red, blue, and purple. The arrows colored in red show the transmission that is in direction of net current, whereas blue arrows demonstrate the reduction in current. And the purple arrow demonstrates the backscattered electrons.
Abbreviations
- DFT:
-
Density functional theory
- NEGF:
-
Non-equilibrium Green’s function
- NDR:
-
Negative differential resistance
- HLG:
-
HOMO–LUMO gap
- DOS:
-
Density of states
- GGA:
-
Generalized gradient approximation
- VNL:
-
Virtual nano lab
References
R.P. Feynman, There is plenty of room at the bottom. Eng. Sci. 23, 22–36 (1960)
H.W. Kroto, J.R. Heath, S.C.R.E.R.F. O’brienCurlSmalley, C60: Buckminsterfullerene. Nature 318, 162–163 (1985)
H. Prinzbach, A. Weiler, P. Landenberger, F. Wahl, J. Worth, L.T. Scott, M. Gelmont, D. Olevano, B.V. Issendorff, Gas-phase production and photoelectron spectroscopy of the smallest fullerene, C20. Nature 407, 60–63 (2000)
M. Otani, T. Ono, K. Hirose, First-principles study of electron transport through C20 cages. Phys. Rev. B 69(12), 121408(R) (2004)
R. He, X.C. Zeng, Electronic structures and electronic spectra of all-boron fullerene B40. Chem. Commun. 51(15), 3185–3188 (2015). https://doi.org/10.1039/c4cc09151e
H.J. Zhai, Y.F. Zhao, W.L. Li, Q. Chen, H. Bai, H.S. Hu, Z.A. Piazza, W.J. Tian, H.G. Lu, Y.B. Wu, Y.W. Mu, G.F. Wei, Z.P. Liu, J. Li, S.D. Li, L.S. Wang, Observation of an all boron fullerene. Nat. Chem. 6, 727–731 (2014)
Z. Yang, Y.L. Ji, G. Lan, L.C. Xu, X. Liu, B. Xu, Design molecular rectifier and photodetector with all boron fullerene. Solid State Commun. 217, 38–42 (2015)
C.X. TangZhang, The hydrogen storage capacity of Sc atoms decorated porous boron fullerene B40: a DFT study. Int. J. Hydrog. Energy 41, 16992–16999 (2016)
J. Wang, T. Yu, Y. Gao, Z. Wang, All-boron fullerene B40: a superatomic structure. Sci. China Mater. 60(12), 1264–1268 (2017)
M. Keyhanian, D. Farmanzadeh, The role of Mn and Fe transition metal atoms mediation on the aniline adsorption by B40 fullerene: a computational investigation. J. Mol. Liq. 249(2019), 111638 (2019)
J. Kaur, R. Kaur, To delve about the charge transport properties of p-block elements doped M@B40 (M = Al, Si, P, S) molecular device. 8th ICCCNT, July 3–5 (Delhi, India, 2017), pp. 1–4
E. Shakerzadeh, Z. Biglari, E. Tahmasebi, M@B40 (M =Li, Na, K) serving as a potential promising novel NLO nanomaterial. Chem. Phys. Lett. 654, 76–80 (2016)
R. Vohra, R.S. Sawhney, J. Kaur, R. Kumar, Adenine based molecular junction as biosensor for detection of toxic phosgene gas. J. Mol. Model 26, 172 (2020)
M. Brandbyge, J.L. Mozos, P. Ordeion, J. Taylor, K. Stokbro, Phys. Rev. B 65, 165401 (2002)
X. Sun, J. Du, L. Zhao, G. Jiang, Water dissociation and H migration on metal decorated B40: a density functional theory (DFT) study. J. Mol. Liq. (2020). https://doi.org/10.1016/j.molliq.2020.113759
Z. Maniei, E. Shakerzadeh, Z. Mahdavifar, The theoretical approach into potential possibility of efficient tNO2 detection via B40 and Li@B40 fullerenes. Chem. Phys. Lett. 691, 360–365 (2018)
S. Cheng, X. Sun, L. Zhao, J. Chen, The interaction of guanine nucleobase with B40 boronspherene. Eur. Phys. J. D 73, 88 (2019)
Y. Zhang, X. Han, X. Cheng, The high capacity hydrogen storage material of Y-doped B40: a theoretical study, Chem. Phys. Lett. 739, 136961 (2020). ISSN 0009-2614, https://doi.org/10.1016/j.cplett.2019.136961.
N. Kosar, F. Ullah, K. Ayub, U. Rashid, M. Imran, M.N. Ahmed, T. Mahmood, Theoretical investigation of halides encapsulated Na@B40 nanocages for potential applications as anodes for sodium-ion batteries. Mater. Sci. Semicond. Process. 121, 105437 (2020)
J. Kaur, R. Kumar, R. Vohra, R. Sawhney, Borospherene molecular junction-based sensor for detecting radium and radon in water. J. Mater. Res. 35(22), 3048–3057 (2020). https://doi.org/10.1557/jmr.2020.205
J. Wang, W. Jiang, W. Xie, J. Wang, Z. Wang, Superatom-assembly induced transition from insulator to semiconductor: a theoretical study. Sci. China Mater. (2018). https://doi.org/10.1007/s40843-018-9329-8
R. Kaur, J. Kaur, The electronic transport properties of B40 fullerenes with chalcogens as anchor atoms. J. Mol. Model. 23(12), 351 (2017). https://doi.org/10.1007/s00894-017-3520-8
R. Kaur, S.B. Narang, D.K.K. Randhawa, Investigating the influence of electrode Miller indices alteration on electronic transport in thiophene-based molecular junctions. J. Mol. Model 24, 63 (2018). https://doi.org/10.1007/s00894-018-3615-x
R. Kaur, N. Kaur, Reconnoitring the current characteristics of the double C20 fullerene molecular device in two probe configuration. J. Mol. Model 23, 255 (2017). https://doi.org/10.1007/s00894-017-3430-9
N. Kaur, R. Kaur, To elucidate the conductive behaviour of transition metals (Mn, Fe, Co, Ni) doped C20 molecular device, 2017 8th International Conference on Computing, Communication and Networking Technologies (ICCCNT) (2017), pp. 1–4. https://doi.org/10.1109/ICCCNT.2017.8203968
J.P. Perdew, K. Burke, M. Ernzerhof, Phys. Rev. Lett. 77, 3865 (1996)
N.D. Lang, Resistance of atomic wires. Phys. Rev. B Condens. Matter Mater. Phys. 52, 5335 (1995)
R. Landauer, Conductance determined by transmission: probes and quantized constriction resistance. J. Phys. Condens. Matter 1, 8099 (1989)
J. Heurich, J.C. Cuevas, W. Wenzel, G. Schon, Electrical transport through single-molecule junctions: from molecular orbitals to conduction channels. Phys. Rev. Lett. 88, 256803 (2002). https://doi.org/10.1103/PhysRevLett.88.256803
J.W. Lawson, C.W. Bauschlieher, Transport in molecular junctions with different metallic contacts. Phys. Rev. B: Condens. Matter Mater. Phys. 74, 125401 (2006)
G.C. Solomon, C. Herrmann, T. Hansen, V. Mujica, M.A. Ratner, Exploring local currents in molecular junctions. Nat. Chem. 2(3), 223–228 (2010). https://doi.org/10.1038/nchem.546
L. Esaki, New phenomenon in narrow germanium p–n junctions. Phys. Rev. 109, 603–604 (1958)
Acknowledgments
The authors acknowledge the university with the Potential for Excellence (UPE) scheme of UGC for providing us the computational facility of Virtual Nano Lab in Guru Nanak Dev University, Amritsar for carrying out this research work. This research work did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Funding
This research does not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
Rajan Vohra came up with the idea and wrote the introduction section. Harleen Kaur performed the necessary modeling and simulations and analyzed the results. Jupinder Kaur interpreted the results and proof-read the manuscript. Ravinder Kumar interpreted the results and edited the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all the authors, the corresponding author states that there is no conflict of interest.
Consent for publication
All authors give their consent.
Consent to participate
All authors give their consent.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Vohra, R., Kaur, H., Kaur, J. et al. Investigation of transport behavior in borospherene-based molecular wire for rectification applications. Journal of Materials Research 37, 360–368 (2022). https://doi.org/10.1557/s43578-021-00364-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/s43578-021-00364-6