Abstract
Microwave (MW) dielectric ceramics are used in numerous electronic components for modern wireless communication systems, including antennas, resonators, capacitors and filters. However, to date, MW ceramics are manufactured by an energy-intensive, conventional high-temperature (> 1000 °C) sintering technology and thus cannot be co-sintered with low melting point and base electrodes (Ag, Al, etc., < 1000 °C), nor directly integrated with polymers (< 200 °C). Cold sintering is able to densify ceramics at < 200 °C via a combination of external pressure and a transient liquid phase, reducing the energy consumed and facilitating greater integration with dissimilar materials. This review outlines the basics of MW ceramics alongside the mechanism of cold sintering. Recent developments in cold sintering of MW ceramics, composites and devices are described, emphasizing new materials and progress towards component/device fabrication. Future prospects and critical issues for advancing cold-sintered MW materials and devices, such as unclear mechanism, low Q × f values and poor mechanical properties, are discussed.
Graphic abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In this review, we present a brief introduction to microwave (MW) ceramics alongside an overview of the mechanisms and state-of-the-art in cold sintering. Subsequently, the review focuses on recent developments in cold sintering MW materials and devices, emphasizing new materials and progress towards component/device fabrication. Finally, the future prospects and critical issues for cold-sintered MW materials and devices are discussed.
Microwave ceramics
MW dielectrics play important roles in the Internet of Things (IoT), fifth-generation (5G) mobile communication technology and global navigation satellite systems (GNSS), as filters, resonators, antennas and substrates [1, 2]. The three selective parameters for MW dielectric materials are relative permittivity (εr), quality factor (Q, often multiplied by the resonant frequency, f0, to give a material constant Q × f) and the temperature coefficient of resonant frequency (TCF or τf).
In conventional electrical circuits, εr defines the energy storage capacity of a material when a potential is applied. In the context of MW ceramics however, it is a measure of the interaction of the electromagnetic (EM) wave with the phonon modes of the crystal structure. Generally speaking, the higher the ionic polarizability of the ions within a crystal structure, the larger the value of εr at MW frequencies. When MWs travel in a dielectric medium, the wavelength is inversely proportional to the \(\sqrt {\varepsilon_{r} }\), according to the equation [2]:
where λd and λ0 is the wavelength in the dielectric and vacuum, respectively, c is the speed of light and ν is frequency. At f0, ν = f0 and λd is approximately the diameter of the resonating body (D), depending on the resonant mode, such that [1, 2]
Circuit miniaturization for low-frequency applications (2–6 GHz), therefore, requires larger εr, whereas higher working frequencies (e.g. mm-wave communication) need smaller values. For 4G and the current generation of 5G (2–6 GHz) telecommunications, ceramics with medium εr (20 < εr < 50) are typically used for resonators and filters in mobile phone networks (e.g. base stations) [1, 2]. As the 5G network expands however, mm-wave technology will dominate and lower values of εr are required with ultralow loss (Q × f > 100,000 GHz).
Q is the reciprocal of the dielectric loss (tanδ) and indicates the quantitative dissipation of the electrical energy within a dielectric. In a MW ceramic, it is a function of the width of the resonant peak, measured at 3 dB below peak height (∆f0) and is given by f0/∆f0. tanδ is the summation of intrinsic and extrinsic losses; intrinsic losses relate to the crystal structure and are described by the interaction of the phonons with the EM field and define the lower limit of tanδ and the upper limit of Q in a defect-free single crystal, whereas extrinsic losses are associated with imperfections in the crystal lattice such as impurities, defects, grain boundaries, pores, microcracks, order–disorder, random crystallite orientation, dislocations, vacancies and dopant atoms [1, 2]. Extrinsic losses can be eliminated or minimized by optimizing material processing. Generally speaking, Q linearly decreases with increasing frequency [1].
The TCF or τf indicates the thermal stability of a resonating body and describes the drift of f0 as a function of temperature. It is given by [1]
where τε and αL are the temperature coefficient of εr and the linear expansion coefficient, respectively.
For base stations, unmetallized ceramics are used within cavities as filters and resonators and are required to have τf = ± 1 ppm/ °C, ultra-high Q × f (> 40, 000 GHz) and 5<τr< 50 depending on the operating frequency. For antennas and radio frequency (RF) substrates which typically have surface metallizations, τf (± 10 ppm/ °C) is relaxed with lower values of Q × f acceptable. τr depends on the application but rarely exceeds 100.
MW ceramics used in co-fired waveguide circuits are divided into four classifications depending on sintering temperature [3]: high-temperature co-fired ceramics (HTCCs, > 1000 °C), low-temperature co-fired ceramics (LTCCs, 700–1000 °C), ultralow-temperature co-fired ceramics (ULTCCs, 400–700 °C) and cold-sintered co-fired ceramics (CSCCs, < 200 °C). HTCCs [4, 5] are typically complex perovskites [6, 7], barium titanates [8], niobates [9, 10], silicates [11,12,13], which cannot be co-fired with base-metal or low melting temperature electrodes (e.g. Ag, Cu, Al) and whose manufacture is energy intensive [14]. Further miniaturization and integration of MW devices have driven the development of LTCCs and ULTCCs, which may be co-fired with base-metal electrodes without melting and interaction [15,16,17,18,19,20]. The family of LTCCs and ULTCCs mainly includes glass–ceramics [21,22,23], tellurates [24, 25], molybdates [26,27,28,29,30,31], vanadates [32,33,34,35], tungstates [36,37,38] and borates [39,40,41].
Despite recent advances, the sintering temperatures of LTCCs and ULTCCs are still much higher than can be withstood by a polymer-based printed circuit boards (PCBs < 200 °C), which inhibits the development of integrated, directly packaged RF devices and systems. CSCCs are a series of ceramics or ceramic composites that can be densified at ultralow temperatures < 200 °C by the cold sintering process (CSP) [3, 42,43,44,45,46,47,48,49], which enables not only co-firing with base-metal electrodes [3, 50, 51] but also PCBs [52] and polymers [43, 53]. Along with CSCCs for RF applications, cold sintering has also been employed to densify ferroelectrics [54,55,56,57,58], piezoelectrics [59,60,61,62,63], thermoelectrics [64], semiconductors [65,66,67,68,69,70], electrolytes [71,72,73,74,75], cathodes [76, 77] and oxides [78,79,80,81,82], which are widely used in many applications, Fig. 1.
Cold sintering
MW ceramics are discussed in detail in section “Microwave Dielectric Materials and Devices by Cold Sintering” but here we present a brief overview of a broader range of functional ceramics that have been shown to cold sinter, alongside a detailed description of the process and its mechanism.
Overview of cold sintering of functional ceramics
Guo et al. [58] demonstrated that ferroelectric BaTiO3 (BT) ceramics could be densified (relative density, ρr ~ 93%) at 180 °C with the help of Ba(OH)2/TiO2 suspension, which was further increased to 95% after post-annealing treatment. More recently, dense BT nanoceramics (ρr ~ 92–95%, grain size ~ 75–150 nm) were successfully cold sintered in a single step at 300 °C for 12 h at 520 MPa with the aid of a molten hydroxide flux (NaOH:KOH = 1:1) [55]. Piezoelectric ceramics, such as lead zirconate titanate (Pb(Zr,Ti)O3, PZT), potassium-sodium niobate (K0.5Na0.5NbO3, KNN) and sodium bismuth titanate (Na0.5Bi0.5TiO3), have been successfully densified by cold sintering with the help of transient liquid-phase (Pb(NO3)2 solution for PZT, NaCl aqueous solution for KNN and Bi(NO3)3/NaOH/TiO2 suspension for NBT [59,60,61,62,63]. Semiconductor V2O5 as well as V2O5-poly(3,4-ethylenedioxythiophene) poly(styrene sulfonate) (PEDOT:PSS) composites have been cold sintered at 120 °C [66]; the electrical conductivity (4.8 × 10−4 S/cm at 25 °C), activation energy (0.25 eV at 25 °C) and Seebeck coefficient (− 990 μV/K at 50 °C) of cold-sintered V2O5 were comparable to conventionally sintered samples and were further increased by the addition of 1–2 vol% PEDOT:PSS. The current status of cold sintering of ZrO2 has been reviewed by Guo et al.; ρr ~ 85% is achieved by a one-step cold-sintered process at 180 °C which increased to ρr ~ 95% with comparable Vickers hardness after annealing at 1100 °C, much lower than conventional sintering process (~ 1400 °C) [79]. The application of cold sintering to solid-state lithium batteries has been reviewed by Liu et al. [46], in which recent progress in different solid electrolytes and electrodes is summarized. Li ion electrolytes and electrodes may be densified but the electrochemical properties of the cold-sintered electrolytes and electrodes required improvement.
The cold sintering process
Sintering is a process which promotes the coalescence of materials (e.g. powders) into a solid with much higher densities than the initial state. The thermodynamic driving force is lowering of the total surface free energy, i.e. [87]
where γA is total surface free energy, γ is surface energy and A is the total specific surface area. During sintering, coarsening of grains contributes to Δγ, whereas the formation of solid–solid interfaces contributes to ΔA. However, ΔG of a sintering process is only a few tens of cal/mol, whereas the formation energies of inorganic solid crystals are typically several thousand cal/mol. A significant energy input is, therefore, required to trigger the sintering process which is usually provided by heating to ~ 1/2 to 3/4 of a material’s melting temperature in °C.
Different sintering methods possess different mass transport mechanisms/routes which ultimately lead to different energy (temperature) requirements. Sintering temperature may be reduced by the application of external pressure (e.g. hot pressing) or the addition of a component that forms a liquid at the sintering temperature. Effectively, these two factors act in combination in cold sintering, leading to a dramatic lowering of the sintering temperature for specific materials [42].
Randall et al. [47] utilized ZnO to study the mechanism(s) of cold sintering, revealing that the gradient of the densification rate varied from 1 to 1/3. They also noticed that the rate of both stages remains almost unchanged with applied pressure, suggesting similar mechanisms are valid over a range of densification conditions. The two different densification rates are attributed to two stages of densification. The first is dominated by particle compaction and rearrangement, whereas the second, with a lower densification rate, relates to a “dissolution–precipitation” mechanism.
Stage 1: particle compaction
At the beginning of stage 1, particles are separated with a solution/void. Densification at this stage is mainly from the compaction (i.e. external pressure pushes particles closer to each other) and rearrangement of particles, as shown in Fig. 2. The solution will also redistribute at this stage due to the combination of external and capillary pressure [88]. Generally speaking, this stage is similar to dry uniaxial pressing but with three extra factors: transient solution, elevated temperature and higher pressure, which help the process individually and collectively.
The transient solution contributes to the particle rearrangement by (i) acting as a lubricant between particles and assists the rearrangement and sliding process, (ii) exerting capillary pressure to pull the particles closer, and (iii) smoothing the particle surface/shape by dissolving sharp edges and contacts to reduce surface energy, further facilitating particle rearrangement and sliding.
The high pressure is the main driving force to the compaction/rearrangement of particles, but in cold sintering, the solubility of particles also increases according to [89]
where N is the mole fraction of the component in the solution, P is the pressure, T is temperature, Vaq is the partial molar volume of component in the solution, Vcr is the partial molar volume of component in the dissolving solid, and R is the universal gas constant. The pressure is concentrated at acicular points of contact and sharp edges (GPa can be realized), which promotes preferential dissolution [90], smoothing particles and helping rearrangement. The elevated temperature is not as critical as the transient solution and high pressure at this stage but increases the solubility and accelerates the dissolution. At the end of stage 1, a relatively rigid skeleton is formed with further particle rearrangement giving only a minor contribution to densification.
Stage 2: dissolution–precipitation and grain coarsening
Stage 2 relies on filling of gaps/voids between grains. In solid-state sintering, mass transport is achieved via two main mechanisms, evaporation–condensation and diffusion through different routes (e.g. surface, grain boundary). In most cases, the contribution from the former is negligible due to low vapour pressure in air, and mass transport is dominated by solid-state diffusion. However, in cold sintering, the temperature is remarkably lower than that of conventional solid state and solid-state diffusion is unlikely. The solid–gas interface is replaced with a solid–liquid interface and dissolution–precipitation is possible, since the energy required for liquid-phase transport is significantly lower than for solid-state diffusion. Dissolution–precipitation is only viable for compositionally sensitive ceramics, if the material can congruently dissolve. For those systems with limited solubility or exhibit incongruent dissolution, densification may still be achieved but this limits properties.
Congruent dissolution
Temperature and pressure both influence the solubility of a material. Within a cold sintering system, the distribution of temperature is generally homogeneous but the pressure is concentrated at the contact points. As a result, the solubility is higher which creates a concentration gradient. Solute is then redistributed by diffusion to other locations with lower concentration (i.e. lower pressure), Fig. 2. The concentration gradient induces the Marangoni effect [91] which causes mass transfer along an interface between two fluids due to a gradient of surface tension, caused by local variations in chemical concentration. As the concentration rises, the solution becomes super-saturated to the point where precipitation begins. With the help of external and capillary pressure to keep the particles in close proximity, dissolution–precipitation initially leads to necking, similar to that observed in solid-state sintering, Fig. 2. As the aqueous solution evaporates, coarsening of particles occurs through precipitation on their surface or alternatively new particles nucleate [65], Fig. 2. An amorphous rather than crystalline phase can form if the solute is strongly bonded with solution [3, 56, 68]. The crystalline phase may, however, be recovered through post-annealing [57, 58]. We note that the gel/glass phase may be beneficial for the cold sintering process by facilitating plastic flow, thereby filling voids.
Incongruent dissolution and limited dissolution
Incongruent dissolution means the composition of the solute in solution does not match that of the solid. An inert surface (passive) layer forms from oxide component(s) which is(are) least soluble. In the case of BaTiO3, Ba2+ ions possess a much higher solubility than Ti4+ [54] and a Ba-depleted layer (i.e. amorphous TiO2) forms on the surface of particles. To overcome this issue, a pre-saturated Ba(OH)2 water solution with TiO2 nanoparticle suspension is introduced [57, 58]. Hydrothermal synthesis takes place at the particle interfaces, densifying the ceramic body, through the formation of a nanocrystalline or amorphous BaTiO3 phase with uniformly distributed Ba and Ti ions. The ρr of the composite is ~ 93% which can be improved to 96% by post-annealing at 900 °C to recrystallize the glass phases [57, 58]. For low solubility compounds such as Pb(Zr,Ti)O3, there is less chance of the formation of a depletion layer and aqueous Pb(NO3)2 has been used as the transient solution to achieve 89% theoretical density [59].
General discussion of the cold sinter process
Comparing congruent with incongruent dissolution, it is evident that higher density (ρ) is much easier to achieve in the former rather than the latter. In some cases, even the external pressure or elevated temperature is not absolutely necessary if the material is highly soluble [42, 92, 93] such as lithium molybdate (Li2MoO4, LMO) and NaCl [42, 88].
Other than the nature of the solvent (e.g. polarity), the solubility may be modified by several factors, including pressure, temperature and particle size (i.e. specific surface area) [94]. The effect of pressure has already been discussed above but temperature follows Le Chatelier’s principle [95] which states that solubility increases with increasing temperature for an endothermic reaction (e.g. NaCl in water), whereas it decreases with the increasing temperature in an exothermic reaction (e.g. CaCO3 in water).
Smaller particles possess higher solubility due to their higher surface free energy according to
where *KA is the solubility constant for the solute particles with the molar surface area A, *KA→0 is the solubility constant for substance with molar surface area tending to zero, γ is the surface tension of the solute particle in the solvent, Am is the molar surface area of the solute, R is the universal gas constant, and T is the absolute temperature. However, this effect only becomes significant when the particle size is at the nanoscale.
Finally, we note the solubility can increase through chemical reaction. When water is used as solvent, the solubility of ZnO is only 0.0004% at room temperature [96]. Yet when hydrochloric acid is used as solvent, the following reaction takes place:
It is therefore reasonable to predict that the outcome of ZnO cold sintered with water or acid as solution will be different. Funahashi et al. [65] reported that the ρr of ZnO after cold sintering is only 65% when pure water or 0.1 mol L−1 acetic acid solution was used, whereas a high ρr of > 90% can be achieved with 1 mol L−1 acetic acid solution.
Ostwald ripening has also been observed in some cold sintering experiments [97]. However, as Ostwald ripening is strictly a surface effect, it does not involve particle movement, and contributes to particle/grain enlargement but not increasing density.
Microwave dielectric materials and devices by cold sintering
Monophase materials
As early as 2014, Kähäri et al. [93] reported the cold sintering of LMO at room temperature with the assistance of deionized water under a pressure of 130 MPa, as shown in the backscattered electron (BSE) images, Fig. 3. ρr of 87–93% was achieved with εr of 4.6–5.2 and Q × f of 10,200–18,500 after post-annealing. In 2015, the same authors reported that ρ and MW properties of LMO could be improved by an increase of powder particle size, applied pressure and post-processing time [98] but there was no significant effect on ρr as a function of the amount of deionized water beyond initial wetting.
BSE images of cold-sintered LMO ceramic samples, (a) sintered at 540 °C, (b) dried at 120 °C and (c) dried at room temperature [93].
Guo et al. [42] at 2016 reported a range of monophase cold-sintered MW ceramics, Fig. 4, including NaCl, LMO, Na2Mo2O7 (NMO), K2Mo2O7, Li2WO4, Na2WO4, BiVO4. With the help of 85% relative humidity, NaCl ceramics could be densified to 90% density at room temperature without applying uniaxial pressure, as shown in Fig. 4b), and was denser than samples sintered at 600 °C and 700 °C (ρr = 84%). Significant grain growth (initially 3 μm to 20–30 μm, Fig. 4b) was observed due to the high aqueous solubility of salt and long holding time. For MW ceramics of low solubility such as LMO (Fig. 4e, ρr ~ 95.7%, εr ~ 5.61, Q × f ~ 30,500), NMO (Fig. 4f, ρr ~ 93.7%, εr ~ 13.4, Q × f ~ 14,900), and K2Mo2O7 (Fig. 4g, ρr ~ 94.1%, εr ~ 9.8, Q × f ~ 16,000), higher temperature (120 °C) and uniaxial pressure (350 MPa) are required.
SEM images of NaCl sintered at (a) room temperature and 75% relative humidity for 24 h, (b) room temperature and 85% relative humidity for 10 h, (c) 600 °C for 50 min and (d) 700 °C for 10 min. Cross-section SEM images of (e) LMO, (f) NMO and (g) K2Mo2O7 ceramics cold sintered at 120 °C and 350 MPa for 15 min [42].
The following year, Guo et al. [3] proposed that the ‘cold sintering offered a new era for ceramic packaging and microwave device development’. To illustrate this point, (Li,Bi)0.5MoO4 (LBMO) was cold sintered at 120 °C, Fig. S1, with ρr of 88.5%, εr of 33.7, Q × f of 2300 and τf of +184 ppm/ °C. The grain size of cold-sintered LBMO was limited to the initial particle size of ceramic powders, since most systems were coarse-grained and do not achieve > 92% density and grain growth is suppressed.
Hong et al. [92, 99] systematically studied the effect of applied pressure and water on cold sintering of NaCl ceramics at room temperature. As applied uniaxial pressure increased, ρr of dry NaCl water increased from 80% (50 MPa) to 99.3% (300 MPa), which is due to the plastic deformation [92]. They found that the addition of 4% water promoted the dissolution–precipitation process, giving a homogenous microstructure, leading to ρr ~ 99.3% and optimal MW properties, εr ~ 5.55, Q × f ~ 49,600 GHz and τf ~ − 173 ppm/ °C at 300 MPa, as plotted in Fig. S2. The same authors [99] also found that ρr and εr decreased from 99.3 to 93.4% and 5.88 to 5.44, respectively, with excess water from 0 to 10%, which was attributed to the volume increase caused by the water evaporation. The highest Q × f (49,600 GHz) was obtained with 4% water, due to the homogenous microstructure.
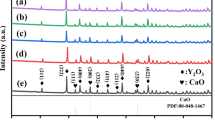
Recently, Wang et al. reported several cold-sintered MW ceramics with 4.7 < εr< 30, such as Na0.5Bi0.5MoO4 (NBMO, ρr ~ 84.9%, εr ~ 20.7, Q × f ~ 1500, τf ~ + 46 ppm/ °C) [100], (Bi0.95Li0.05)(V0.9Mo0.1)O4 (BLVMO, ρr ~ 73%, εr ~ 30, Q × f ~ 1300, τf ~ + 61 ppm/ °C) [101], KCl (ρr ~ 98%, εr ~ 4.74, Q × f ~ 7738, τf ~ − 149 ppm/ °C) [101], K2MoO4 (KMO, ρr ~ 100%, εr ~ 6.37, Q × f ~ 26,500, τf ~ − 70 ppm/ °C) [101], and AgNaMoO4 (ANMO, ρr ~ 90.8%, εr ~ 9.3, Q × f ~ 7078, τf ~ − 120 ppm/ °C) [101], as listed in Table S1. The majority of the above are based on molybdates but recently, lower cost LiMgPO4 (LMP, ρr ~ 93%, εr ~ 6.5, Q × f ~ 16,000) MW ceramics were also successfully cold sintered [102], Fig. 5. In this work, with the increase of cold sintering temperature and time, ρr and εr were enhanced (Fig. 5a, b) and slightly higher than conventionally sintered samples (ρr ~ 92% and εr ~ 6.4). In addition, no impurity or secondary phase was observed during the cold sintering process, as shown in Fig. 5d, e.
ρ and ρr of LMP ceramics as a function of (a) sintering temperature and (b) sintering time. (c) The schematic crystal structure of LMP. XRD patterns of LMP ceramics cold sintered at different (d) sintering temperatures and (e) sintering times. (f) Rietveld refinement of cold-sintered LMP ceramics [102].
Composite materials
Despite promising values of Q × f and εr for MW applications, no monophasic ceramics have to date been shown to be temperature stable (τf ~ 0). Kähäri et al. [98] attempted to tune τf and reported cold-sintered LMO ceramics with the addition of 10 vol% TiO2 and BaTiO3 at 120 °C, respectively, as shown in Fig. 6, which led to the increase of εr and loss tangent (Table S2). The effect of different amounts of TiO2 on the microstructure and MW dielectric properties of LMO was also investigated in a related study [103]. εr increased from 5.1 (0 vol% TiO2) to 10.1 (30 vol% TiO2), as listed in Table S3 [98], but τf (+20 ppm/oC) remained stubbornly, high even with 20 vol% TiO2 (Table S3) [103].
BSE images of LMO ceramic composites with an addition of (a) 10 vol% of TiO2 and (b) 10 vol% of BaTiO3 [98].
In 2016, Guo et al. [43] proposed that cold sintering bridged the processing temperature gap of ceramic and polymer materials, schematically shown in Fig. 7a and reported several series of ceramic-polymer composites (LMO–(–C2F4–)n (PTFE), electrolyte Li1.5Al0.5Ge1.5(PO4)3–(–CH2CF2–)x[–CF2CF(CF3)–]y (PVDF-HFP), semiconductor V2O5-PEDOT:PSS). High density (ρr > 93%) and homogenous microstructure were obtained for LMO-xPTFE (0 ≤ x ≤ 70 vol%), as shown in the BSE images, Fig. 7b, c. Furthermore, by integrating different electrical and mechanical features of both ceramic and polymer, novel composites with integrated properties could be designed and achieved, Fig. 8. As PTFE concentration increased, εr of LMO-xPTFE decreased from 5.8 to 2.9, τf shifted from − 170 to − 7.2 ppm/°C, elastic modulus and shear modulus decreased, while no obvious deterioration was observed in Q × f, Fig. 8b–f.
(a) Schematic of cold-co-sintered ceramic-polymer composites with different amounts of polymer. BSE images of (b) 0.9LMO-0.1PTFE and (c) 0.4LMO-0.6PTFE composites cold sintered at 120 °C and 350 MPa for 20 min [43].
(a) Schematic of integrated material properties by balancing ceramic/polymer ratio. Electrical and mechanical properties of LMO-PTFE composites as a function of PTFE volume fraction: (b) εr, (c) (Q × f, (d) TCF (τf), (e) elastic modulus and (f) shear modulus [43].
Inspired by Guo et al., many novel MW dielectric ceramic–ceramic/polymer composites were further developed, including LBMO-PTFE [3], Al2SiO5–NaCl [104], LMO-MnZn [105], NBMO-LMO [100], LMO-BaFe12O19 [106], LMO-Mg2SiO4 [107], BLVMO-NMO [50, 101], CaTiO3(CTO)-KMO [51], Bi2Mo2O9(BMO)-KMO [52], garnet-LMO/NaCl [108], and LMP-CTO-KMO [102]. Most pertinently however, Wang, Reaney and co-workers developed several MW ceramic composites with near-zero τf which exhibited a wide range of εr, such as 8 wt%CTO–92 wt%KMO (Fig. S3, εr ~ 8.5) [51], 50 wt%LMP–10 wt%CTO–40 wt%KMO (εr ~ 9.1) [102], 65 wt%CaSnSiO5–35 wt%KMO (εr ~ 9.2) [109], 80 wt%NBMO–20 wt%LMO (εr ~ 17.4) [100], 90 wt%BMO–10 wt%KMO (εr ~ 31) [52], and 80 wt%BLVMO–20 wt%NMO (εr ~ 40) [50, 101]. All compositions could be co-fired with polymers and Ag/base-metal electrode without interaction (Fig. S3k,l) [51, 109] and had the potential to be used in antennas, resonators, filters and capacitors.
The fabrication of ceramic–ceramic layered composites is difficult by conventional sintering technology, due to interfacial reaction, delamination and wrapping, caused by inequivalent densification rates and differential thermal expansion on cooling. These issues are completely resolved by cold sintering either due to the ultralow sintering temperatures or the absence of lateral shrinkage. Wang et al. demonstrated using NBMO-xLMO (Fig. 9A), a three-layered macroscopic ceramic–ceramic graded dielectric composite for the fabrication of MW dielectric GRaded INdexed (GRIN) lenses, Fig. 9B [100]. The graded composite had a high density with no delamination or wrapping.
(A) BSE and SEM images of cold-sintered (a, b) 90 wt%NBMO–10 wt%LMO and (c, d) 20 wt%NBMO–80 wt%LMO composite samples; (B) (a) schematic illustration and (b) optical picture of the NBMO-LMO three-layered composite ceramics [100].
To our knowledge, all MW ceramics and composites fabricated to date by cold sintering are summarized in Table 1 which illustrates the range of εr, τf and Q × f values which can be achieved and indicates their great promise for fabrication of RF devices.
RF devices
To demonstrate the potential of cold sintering for RF applications, different devices have been designed and fabricated. In 2016, Baker et al. printed LMO monolithic capacitors on PET/Ni foil substrates with Ag internal electrodes [53], as shown in Fig. 10, thereby demonstrating a facile and flexible method for the production of single and multilayer capacitors using cold sintering, Fig. S4 [3].
Photograph of (A) printed LMO capacitors on PET films and (B) printed LMO capacitors on Ni foils; (c) cross-sectional SEM images of a cold-sintered single-layered LMO capacitor [53].
Simultaneously, the first microstrip patch antenna was designed and fabricated using a cold-sintered LMO disk as a substrate, Fig. 11 [112]. Lateral dimensions were controlled by the mould, simplifying the design/modelling of the antenna performance. However, due to high relative humidity (80%) and water absorption, a lower f0 and a reduced antenna efficiency were observed compared to the simulated results, which was subsequently resolved using a silicone conformal coating.
(A) Layout and cross section of the designed antenna and (B) image of a fabricated circular LMO patch antenna [112].
Wang et al. also designed and simulated a novel dielectric GRIN lens based on cold-sintered NBMO-xLMO [100] and BLVMO-xNMO [101] ceramic systems (Fig. S5) which exhibited good aperture efficiencies of 78% and 70%, respectively. Furthermore, BLVMO-NMO-based multilayer ceramic capacitors (MLCCs) were successfully cold sintered at 150 °C with Ag internal electrodes without evidence of interfacial reaction, delamination and wrapping (Fig. 12); [50] the dielectric properties of which (εr = 39, TCC ≈ ± 0.01%, tanδ = 0.01 at 1 MHz) corresponded to a C0G designation (± 30 ppm/ °C) in the Electronic Industry Alliance codes.
(a) SEM image for the cross section of BLVMO-NMO MLCCs; EDS elemental mapping: (b) elemental layered image, (c) Ag, (d) Na, (e) Bi, (f) Mo, (g) V and (h) O [50].
Following on from Kohari et al. Wang et al. [51] utilized a temperature stable cold-sintered composition, CTO-KMO, to design and fabricated a microstrip patch antenna (40.0 mm × 40.0 mm × 1.4 mm), with copper tape (27.5 mm × 23.0 mm × 0.05 mm) as the radiation element and ground plane, as shown in the inset of Fig. 13a. The microstrip patch antenna operated at a centre operating frequency of ~ 2.5 GHz (Fig. 13a) and the realized gain was 2.73 dBi at 2.5 GHz, leading to a radiation efficiency of 62% [51].
Measured (a) S11 and (b) radiation pattern of the 8% wt%CTO-92 wt%KMO microstrip antenna. The picture of fabricated microstrip antenna is given in the inset of (a) [51].
A 5G prototype microstrip patch antenna was further fabricated by Li et al. [109] using cold-sintered CaSnSiO5-KMO composite ceramics, as shown in the inset of Fig. S6a, which exhibited a centre operating frequency of 5.2 GHz with good impedance match and a 144 MHz bandwidth (− 10 dB) (Fig. S6a). A high total efficiency of 88.4% was obtained in this antenna at the frequency of 5.2 GHz with an antenna gain of 5.7 dBi (Fig. S6c), again suggesting CaSnSiO5-KMO compositions are suitable for antenna applications.
Most importantly from a manufacturing perspective, Wang et al. [52] have demonstrated that cold-sintered, temperature stable 0.9BMO–0.1KMO (30 × 30 × 7 mm, εr ~ 31) could be directly pressed onto PCBs (TM < 200 °C) with a satellite navigation antenna subsequently fabricated using the standard Cu metallization as the ground plane, as shown in Fig. 14A [52]. No chemical reaction/delamination was observed at the interface between ceramic and Cu layers, as shown in the SEM images and EDS elemental line-scans in Fig. 14B. An S11 of − 10 dB and a bandwidth of 59 MHz was measured in the antenna with an axial ratio < 3 dB (Fig. 14C), which covered the desired frequency bands 1561 MHz for BeiDou and 1575 MHz for GPS/Galileo with high efficiencies of 87–88%, as shown in Fig. 14D. This latter contribution demonstrates the potential of cold sintering for the fabrication of low-cost integrated ceramic-polymer substrate technology, opening up new design space for a plethora of directly integrated devices ranging from antennas to dielectric resonator arrays. In addition to the reduction in manufacturing costs, the much lower temperature with cold sintering enables precise control of lateral dimensions of the ceramic body, which is a great asset in manufacturing since f0 is determined based on the mould dimensions (no lateral shrinkage) and the powder charge without the need for post-fabrication tuning, a weakness in conventional processing.
(A) Schematic diagram of cold sintering ceramic substrate for antennas; (B) SEM images and EDS elemental line-scans of a cross section of cold-sintered BMO-KMO on a Cu-metallized PCB; (C) measured S11 curve of the antenna and (D) measured antenna performance of the fabricated ceramic antenna at two frequency bands [52].
Summary and perspectives
In this contribution, the underpinning principles of MW ceramic technology and cold sintering have been reviewed. MW ceramics and RF devices (substrates, resonators, filters, etc.) are widely used in wireless and satellite communication technology, recently accelerated by the deployment of 5G and ultimately mm-wave networks. They are manufactured typically at high temperatures and are expensive due to the large amount of energy required for densification in addition to the high raw materials costs. Cold sintering, particularly for congruent dissolving ceramics and composites, offers, through its unique “dissolution–precipitation” process, a feasible and sustainable technology to realize ultralow-temperature and low-carbon manufacturing of MW devices. Temperature stable cold-sintered compositions are now available with 2.9 < εr< 48 and Q × f suitable for a wide range of applications. Furthermore, the unique compatibility that cold sintering offers with polymers and metals creates a hitherto, unexplored design space for component fabrication and manufacturing, such as directly integrated ceramics on metallized PCBs and new functionality through graded structures. However, several critical issues need to be addressed in future:
-
(1)
Although the cold sintering process has been investigated and discussed by Randall et al. [47], its mechanism(s) still require(s) further clarification. At the moment, the prevailing view is that there are strong similarities with liquid-phase sintering but we acknowledge that the exact processes that occur inside the die at temperature and pressure still remain to be elucidated.
-
(2)
Most cold-sintered MW ceramics and composites are molybdates or molybdate based. The high cost of Mo means that any economic benefit from reduction of energy in manufacturing may be lost in the high cost of raw material. Industry would like to see drop-in replacements for conventional ceramics based on e.g. Al2O3, TiO2, CaTiO3 and SrTiO3. However, these materials are poorly soluble and require complex cold-sintered approaches that do not readily maintain high Q × f values. Cold-sintered compositions based on phosphates look to be a potential way to decrease cost while retaining usable properties, with initial success recently demonstrated by Wang et al. [102].
-
(3)
Although many MW ceramics and composites can be densified by cold sintering, most of their Q × f values are quite low compared to samples sintered conventionally. Q × f for resonators is required to be > 40,000 GHz for most applications but to date this seems beyond the reach of cold-sintered, temperature stable compositions. Randall et al. [47] attribute this phenomenon to the complex microstructure of grain boundary (amorphous phase, interfacial films, etc.) and limited grain growth (small grain size). It is critical, therefore, to modify the process of cold sintering to eliminate interfacial amorphous phase and promote grain growth so that Q × f may be optimized. There are nascent related technologies based on hybrid techniques such cold/flash sintering or MW-assisted cold sintering that could, if successfully developed, not only expand the range of cold-sintered ceramics but also improve the interparticle interface and thus Q × f.
-
(4)
Sensitivity to atmospheric moisture due to the partially soluble nature of cold-sintered ceramics may also pose issues for long-term usage. In addition, mechanical properties (and thus handling characteristics) have been reported to be significantly worse than their conventionally sintered counterparts. Humidity sensitivity may well be resolved through a low-cost polymer encapsulation process [112] but how to mitigate against low mechanical strength remains unclear and could limit cold sintering to integrated structures on PCBs which would supply superior handling characteristics.
-
(5)
Many RF devices (antennas, resonators, filters, etc.) have relatively simple geometries (rectangular blocks or cylinders). Further work required to understand and control the cold-sintered process in more complex pressed or moulded shapes. In principle however, if a shape can be uniaxially pressed prior to conventional sintering, then it is feasible that it can fabricated by cold sintering.
References
I.M. Reaney, D. Iddles, Microwave dielectric ceramics for resonators and filters in mobile phone networks. J. Am. Ceram. Soc. 89(7), 2063 (2006)
M. Sebastian, R. Ubic, H. Jantunen, Low-loss dielectric ceramic materials and their properties. Int. Mater. Rev. 60(7), 392 (2015)
J. Guo, A.L. Baker, H. Guo, M. Lanagan, C.A. Randall, Cold sintering process: a new era for ceramic packaging and microwave device development. J. Am. Ceram. Soc. 100(2), 669 (2017)
R. Cava, Dielectric materials for applications in microwave communications. J. Mater. Chem. 11(1), 54 (2001)
H. Ouchi, S. Kawashima, Dielectric ceramics for microwave application. Jpn. J. Appl. Phys. 24(S2), 60 (1985)
I.M. Reaney, E.L. Colla, N. Setter, Dielectric and structural characteristics of Ba-and Sr-based complex perovskites as a function of tolerance factor. Jpn. J. Appl. Phys. 33(7R), 3984 (1994)
E. Colla, I. Reaney, N. Setter, Effect of structural changes in complex perovskites on the temperature coefficient of the relative permittivity. J. Appl. Phys. 74(5), 3414 (1993)
R. Ubic, I. Reaney, W. Lee, Microwave dielectric solid–solution phase in system BaO–Ln203–Ti02 (Ln = lanthanide cation). Int. Mater. Rev. 43(5), 205 (1998)
R.C. Pullar, The synthesis, properties, and applications of columbite niobates (M2 + Nb2O6): a critical review. J. Am. Ceram. Soc. 92(3), 563 (2009)
L.X. Pang, D. Zhou, Modification of NdNbO4 microwave dielectric ceramic by Bi substitutions. J. Am. Ceram. Soc. 102(5), 2278 (2019)
Z. Tan, K. Song, H.B. Bafrooei, B. Liu, J. Wu, J. Xu, H. Lin, D. Wang, The effects of TiO2 addition on microwave dielectric properties of Y3MgAl3SiO12 ceramic for 5G application. Ceram. Int. 46(10), 15665 (2020)
Q. Lin, K. Song, B. Liu, H.B. Bafrooei, D. Zhou, W. Su, F. Shi, D. Wang, H. Lin, I.M. Reaney, Vibrational spectroscopy and microwave dielectric properties of AY2Si3O10 (A = Sr, Ba) ceramics for 5G applications. Ceram. Int. 46(1), 1171 (2020)
Z. Song, K. Song, B. Liu, P. Zheng, H. BarzegarBafrooei, W. Su, H. Lin, F. Shi, D. Wang, I. Reaney, Temperature-dependent dielectric and Raman spectra and microwave dielectric properties of gehlenite-type Ca2Al2SiO7 ceramics. Int. J. Appl. Ceram. Technol. 17(2), 771 (2020)
T. Ibn-Mohammed, C.A. Randall, K.B. Mustapha, J. Guo, J. Walker, S. Berbano, S.C.L. Koh, D. Wang, D.C. Sinclair, I.M. Reaney, Decarbonising ceramic manufacturing: a techno-economic analysis of energy efficient sintering technologies in the functional materials sector. J. Eur. Ceram. Soc. 39(16), 5213 (2019)
M.T. Sebastian, H. Wang, H. Jantunen, Low temperature co-fired ceramics with ultra-low sintering temperature: a review. Curr. Opin. Solid State Mater. Sci. 20(3), 151 (2016)
M.T. Sebastian, H. Jantunen, Low loss dielectric materials for LTCC applications: a review. Int. Mater. Rev. 53(2), 57 (2008)
J. Zhou, Towards rational design of low-temperature co-fired ceramic (LTCC) materials. J. Adv. Ceram. 1(2), 89 (2012)
H. Jantunen, T. Kangasvieri, J. Vähäkangas, S. Leppävuori, Design aspects of microwave components with LTCC technique. J. Eur. Ceram. Soc. 23(14), 2541 (2003)
H. Yu, J. Liu, W. Zhang, S. Zhang, Ultra-low sintering temperature ceramics for LTCC applications: a review. J. Mater. Sci. 26(12), 9414 (2015)
D. Zhou, L.X. Pang, D.W. Wang, C. Li, B.B. Jin, I.M. Reaney, High permittivity and low loss microwave dielectrics suitable for 5G resonators and low temperature co-fired ceramic architecture. J. Mater. Chem. C 5(38), 10094 (2017)
H.P. Braun, A. Mehmood, M. Hovhannisyan, H. Zhang, D.S.B. Heidary, C. Randall, M.T. Lanagan, R. Jakoby, I.M. Reaney, M. Letz, Microwave properties and structure of La–Ti–Si–B–O glass–ceramics for applications in GHz electronics. J. Eur. Ceram. Soc. 37(5), 2137 (2017)
H. Yu, K. Ju, K. Wang, A novel glass–ceramic with ultra-low sintering temperature for LTCC application. J. Am. Ceram. Soc. 97(3), 704 (2014)
I.J. Induja, M.T. Sebastian, Microwave dielectric properties of SnO-SnF2-P2O5 glass and its composite with alumina for ULTCC applications. J. Am. Ceram. Soc. 100(6), 2632 (2017)
M. Valant, D. Suvorov, Glass-free low-temperature cofired ceramics: calcium germanates, silicates and tellurates. J. Am. Ceram. Soc. 24(6), 1715 (2004)
M. Udovic, M. Valant, D. Suvorov, Dielectric characterisation of ceramics from the TiO2–TeO2 system. J. Eur. Ceram. Soc. 21(10–11), 1735 (2001)
R. Gheisari, H. Chamberlain, G. Chi-Tangyie, S. Zhang, A. Goulas, C.-K. Lee, T. Whittaker, D. Wang, A. Ketharam, A. Ghosh, Multi-material additive manufacturing of low sintering temperature Bi2Mo2O9 ceramics with Ag floating electrodes by selective laser burnout. Virt. Phys. Prototyping J. 15(2), 133 (2020)
D. Zhou, L.-X. Pang, D.-W. Wang, H.-H. Guo, F. Yang, Z.-M. Qi, C. Li, B.-B. Jin, I.M. Reaney, Crystal structure, impedance and broadband dielectric spectra of ordered scheelite-structured Bi (Sc1/3Mo2/3)O4 ceramic. J. Eur. Ceram. Soc. 38(4), 1556 (2018)
L.X. Pang, D. Zhou, D.W. Wang, J.X. Zhao, W.G. Liu, Z.X. Yue, I.M. Reaney, Temperature stable microwave dielectrics ceramics with ultra-low sintering temperature. J. Am. Ceram. Soc. 101(5), 1806 (2018)
D. Zhou, J. Li, L.-X. Pang, D.-W. Wang, I.M. Reaney, Reaney: novel water insoluble (NaxAg2−x)MoO4 (0 ≤ x ≤ 2) microwave dielectric ceramics with spinel structure sintered at 410 degrees. J. Mater. Chem. C 5(24), 6086 (2017)
S.-Z. Hao, D. Zhou, F. Hussain, W.-F. Liu, J.-Z. Su, D.-W. Wang, Q.-P. Wang, Z.-M. Qi, C. Singh, S. Trukhanov, Structure, spectral analysis and microwave dielectric properties of novel x (NaBi)0.5MoO4-(1-x)Bi2/3MoO4 (x = 0.2 ∼ 0.8) ceramics with low sintering temperatures. J. Eur. Ceram. Soc. 40(10), 3569 (2020)
A. Goulas, G. Chi-Tangyie, D. Wang, S. Zhang, A. Ketharam, B. Vaidhyanathan, I.M. Reaney, D.A. Cadman, W.G. Whittow, J.Y.C. Vardaxoglou, Additively manufactured ultra-low sintering temperature, low loss Ag2Mo2O7 ceramic substrates. J. Eur. Ceram. Soc. 41, 394–401 (2020)
H.H. Guo, D. Zhou, W.F. Liu, L.X. Pang, D.W. Wang, J.Z. Su, Z.M. Qi, Microwave dielectric properties of temperature-stable zircon-type (Bi, Ce)VO4 solid solution ceramics. J. Am. Ceram. Soc. 103(1), 423 (2020)
D. Zhou, L.-X. Pang, D.-W. Wang, I.M. Reaney, BiVO4 based high k microwave dielectric materials: a review. J. Mater. Chem. C 6(35), 9290 (2018)
D. Zhou, D. Guo, W.-B. Li, L.-X. Pang, X. Yao, D.-W. Wang, I.M. Reaney, Novel temperature stable high-εr microwave dielectrics in the Bi2O3–TiO2–V2O5 system. J. Mater. Chem. C 4(23), 5357 (2016)
J.L. Di Zhou, L.-X. Pang, G.-H. Chen, Z.-M. Qi, D.-W. Wang, I.M. Reaney, Crystal structure, infrared spectra, and microwave dielectric properties of temperature-stable zircon-type (Y, Bi)VO4 solid-solution ceramics. ACS Omega 1(5), 963 (2016)
H.-D. Xie, H.-H. Xi, C. Chen, D. Zhou, Microwave dielectric properties of two low temperature sintering ceramics in the PbO–WO3 binary system. Ceram. Int. 41(8), 10287 (2015)
D. Zhou, C.A. Randall, L.X. Pang, H. Wang, J. Guo, G.Q. Zhang, X.G. Wu, L. Shui, X. Yao, Microwave dielectric properties of Li2WO4 ceramic with ultra-low sintering temperature. J. Am. Ceram. Soc. 94(2), 348 (2011)
J. Guo, D. Zhou, S.L. Zou, H. Wang, L.X. Pang, X. Yao, Microwave dielectric ceramics Li2MO4 − TiO2 (M = Mo, W) with low sintering temperatures. J. Am. Ceram. Soc. 97(6), 1819 (2014)
X. Chen, W. Zhang, B. Zalinska, I. Sterianou, S. Bai, I.M. Reaney, Low sintering temperature microwave dielectric ceramics and composites based on Bi2O3–B2O3. J. Am. Ceram. Soc. 95(10), 3207 (2012)
D. Zhou, L.-X. Pang, D.-W. Wang, Z.-M. Qi, I.M. Reaney, High quality factor, ultralow sintering temperature Li6B4O9 microwave dielectric ceramics with ultralow density for antenna substrates. ACS Sustain. Chem. Eng. 6(8), 11138 (2018)
X.G. Wu, H. Wang, Y.H. Chen, D. Zhou, Synthesis and microwave dielectric properties of Zn3B2O6 ceramics for substrate application. J. Am. Ceram. Soc. 95(6), 1793 (2012)
J. Guo, H. Guo, A.L. Baker, M.T. Lanagan, E.R. Kupp, G.L. Messing, C.A. Randall, Cold sintering: a paradigm shift for processing and integration of ceramics. Angew. Chem. Int. Ed. Engl. 55(38), 11457 (2016)
J. Guo, S.S. Berbano, H. Guo, A.L. Baker, M.T. Lanagan, C.A. Randall, Cold sintering process of composites: bridging the processing temperature gap of ceramic and polymer materials. Adv. Funct. Mater. 26(39), 7115 (2016)
J.-P. Maria, X. Kang, R.D. Floyd, E.C. Dickey, H. Guo, J. Guo, A. Baker, S. Funihashi, C.A. Randall, Cold sintering: current status and prospects. J. Mater. Res. 32(17), 3205 (2017)
J. Guo, X. Zhao, T. Herisson De Beauvoir, J.-H. Seo, S.S. Berbano, A.L. Baker, C. Azina, C.A. Randall, Recent progress in applications of the cold sintering process for ceramic-polymer composites. Adv. Funct. Mater. 28(39), 1801724 (2018)
Y. Liu, Q. Sun, D. Wang, K. Adair, J. Liang, X. Sun, Development of the cold sintering process and its application in solid-state lithium batteries. J. Power Sources. 393, 193 (2018)
J. Guo, R. Floyd, S. Lowum, J.-P. Maria, T. Herisson de Beauvoir, J.-H. Seo, C.A. Randall, Cold sintering: progress, challenges, and future opportunities. Annu. Rev. Mater. Res. 49(1), 275 (2019)
T. Yu, J. Cheng, L. Li, B. Sun, X. Bao, H. Zhang, Current understanding and applications of the cold sintering process. Front. Chem. Sci. Eng. 13(4), 654 (2019)
S. Grasso, M. Biesuz, L. Zoli, G. Taveri, A.I. Duff, D. Ke, A. Jiang, M.J. Reece, A review of cold sintering processes. Adv. Appl. Ceram. 119(3), 115 (2020)
D. Wang, D. Zhou, K. Song, A. Feteira, C.A. Randall, I.M. Reaney, Cold-sintered C0G multilayer ceramic capacitors. Adv. Electron. Mater. 5(7), 1900025 (2019)
D. Wang, S. Zhang, G. Wang, Y. Vardaxoglou, W. Whittow, D. Cadman, D. Zhou, K. Song, I.M. Reaney, Cold sintered CaTiO3-K2MoO4 microwave dielectric ceramics for integrated microstrip patch antennas. Appl. Mater. Today 18, 100519 (2020)
D. Wang, B. Siame, S. Zhang, G. Wang, X. Ju, J. Li, Z. Lu, Y. Vardaxoglou, W. Whittow, D. Cadman, S. Sun, D. Zhou, K. Song, I.M. Reaney, Direct integration of cold sintered, temperature-stable Bi2Mo2O9-K2MoO4 ceramics on printed circuit boards for satellite navigation antennas. J. Eur. Ceram. Soc. 40(12), 4029 (2020)
A. Baker, H. Guo, J. Guo, C. Randall, D.J. Green, Utilizing the cold sintering process for flexible-printable electroceramic device fabrication. J. Am. Ceram. Soc. 99(10), 3202 (2016)
J.-P. Ma, X.-M. Chen, W.-Q. Ouyang, J. Wang, H. Li, J.-L. Fang, Microstructure, dielectric, and energy storage properties of BaTiO3 ceramics prepared via cold sintering. Ceram. Int. 44(4), 4436 (2018)
K. Tsuji, A. Ndayishimiye, S. Lowum, R. Floyd, K. Wang, M. Wetherington, J.-P. Maria, C.A. Randall, Single step densification of high permittivity BaTiO3 ceramics at 300 °C. J. Eur. Ceram. Soc. 40(4), 1280 (2020)
H. Guo, A. Baker, J. Guo, C.A. Randall, D. Johnson, Cold sintering process: a novel technique for low-temperature ceramic processing of ferroelectrics. J. Am. Ceram. Soc. 99(11), 3489 (2016)
H. Guo, A. Baker, J. Guo, C.A. Randall, Protocol for ultralow-temperature ceramic sintering: an integration of nanotechnology and the cold sintering process. ACS Nano 10(11), 10606 (2016)
H. Guo, J. Guo, A. Baker, C.A. Randall, Hydrothermal-assisted cold sintering process: a new guidance for low-temperature ceramic sintering. ACS Appl. Mater. Interfaces. 8(32), 20909 (2016)
D. Wang, H. Guo, C.S. Morandi, C.A. Randall, S. Trolier-McKinstry, Cold sintering and electrical characterization of lead zirconate titanate piezoelectric ceramics. APL Mater. 6(1), 016101 (2018)
M. Chi, W. Ma, J. Guo, J. Wu, T. Li, S. Wang, P. Zhang, Effect of NaCl on the microstructure and electrical properties of prepared by cold sintering process. J. Mater. Sci. 30(24), 21435–21443 (2019)
H. Huang, J. Tang, J. Liu, Preparation of Na0.5Bi0.5TiO3 ceramics by hydrothermal-assisted cold sintering. Ceram. Int. 45(6), 6753 (2019)
J. Ma, H. Li, H. Wang, C. Lin, X. Wu, T. Lin, X. Zheng, X. Yu, Composition, microstructure and electrical properties of ceramics fabricated by cold sintering assisted sintering. J. Eur. Ceram. Soc. 39(4), 986 (2019)
M. Nelo, T. Siponkoski, H. Kähäri, K. Kordas, J. Juuti, H. Jantunen, Upside - down composites: fabricating piezoceramics at room temperature. J. Eur. Ceram. Soc. 39(11), 3301 (2019)
S. Funahashi, H. Guo, J. Guo, A.L. Baker, K. Wang, K. Shiratsuyu, C.A. Randall, Cold sintering and co-firing of a multilayer device with thermoelectric materials. J. Am. Ceram. Soc. 100(8), 3488 (2017)
S. Funahashi, J. Guo, H. Guo, K. Wang, A.L. Baker, K. Shiratsuyu, C.A. Randall, Demonstration of the cold sintering process study for the densification and grain growth of ZnO ceramics. J. Am. Ceram. Soc. 100(2), 546 (2017)
J. Guo, H. Guo, D.S.B. Heidary, S. Funahashi, C.A. Randall, Semiconducting properties of cold sintered V2O5 ceramics and Co-sintered V2O5-PEDOT:PSS composites. J. Eur. Ceram. Soc. 37(4), 1529 (2017)
J. Guo, B. Legum, B. Anasori, K. Wang, P. Lelyukh, Y. Gogotsi, C.A. Randall, Cold sintered ceramic nanocomposites of 2D MXene and zinc oxide. Adv. Mater. 30(32), e1801846 (2018)
D.S.B. Heidary, J. Guo, J.-H. Seo, H. Guo, R. Rajagopalan, C.A. Randall, Microstructures and electrical properties of V2O5 and carbon-nanofiber composites fabricated by cold sintering process. Jpn. J. Appl. Phys. 57(2), 25702 (2018)
S.H. Bang, K. Tsuji, A. Ndayishimiye, S. Dursun, J.H. Seo, S. Otieno, C.A. Randall, Toward a size scale-up cold sintering process at reduced uniaxial pressure. J. Am. Ceram. Soc. 103(4), 2322 (2020)
M.Y. Sengul, J. Guo, C.A. Randall, A.C.T. van Duin, Water-mediated surface diffusion mechanism enables the cold sintering process: a combined computational and experimental study. Angew Chem. Int. Ed. Engl. 58(36), 12420 (2019)
S.S. Berbano, J. Guo, H. Guo, M.T. Lanagan, C.A. Randall, Randall: cold sintering process of Li1.5Al0.5Ge1.5(PO4)3 solid electrolyte. J. Am. Ceram. Soc. 100(5), 2123 (2017)
H. Leng, J. Huang, J. Nie, J. Luo, Cold sintering and ionic conductivities of solid electrolytes. J. Power Sources. 391, 170 (2018)
W. Lee, C.K. Lyon, J.H. Seo, R. Lopez-Hallman, Y. Leng, C.Y. Wang, M.A. Hickner, C.A. Randall, E.D. Gomez, Ceramic–salt composite electrolytes from cold sintering. Adv. Funct. Mater. 29(20), 1807812 (2019)
H. Nakaya, M. Iwasaki, T.H. de Beauvoir, C.A. Randall, Applying cold sintering process to a proton electrolyte material: CsH2PO4. J. Eur. Ceram. Soc. 39(2–3), 396 (2019)
T.H. Zaengle, A. Ndayishimiye, K. Tsuji, Z. Fan, S.H. Bang, J. Perini, S.T. Misture, C.A. Randall, Single-step densification of nanocrystalline CeO2 by the cold sintering process. J. Am. Ceram. Soc. 103(5), 2979 (2020)
J.-H. Seo, J. Guo, H. Guo, K. Verlinde, D.S.B. Heidary, R. Rajagopalan, C.A. Randall, Cold sintering of a Li-ion cathode: LiFePO4-composite with high volumetric capacity. Ceram. Int. 43(17), 15370 (2017)
J.-H. Seo, K. Verlinde, J. Guo, D.S.B. Heidary, R. Rajagopalan, T.E. Mallouk, C.A. Randall, Cold sintering approach to fabrication of high rate performance binderless LiFePO4 cathode with high volumetric capacity. Scripta Materialia. 146, 267 (2018)
H. Guo, T.J.M. Bayer, J. Guo, A. Baker, C.A. Randall, Cold sintering process for 8 mol%Y2O3-stabilized ZrO2 ceramics. J. Eur. Ceram. Soc. 37(5), 2303 (2017)
H. Guo, T.J.M. Bayer, J. Guo, A. Baker, C.A. Randall, Current progress and perspectives of applying cold sintering process to ZrO2-based ceramics. Scripta Materialia. 136, 141 (2017)
H. Guo, J. Guo, A. Baker, C.A. Randall, Cold sintering process for ZrO2-based ceramics: significantly enhanced densification evolution in yttria-doped ZrO2. J. Am. Ceram. Soc. 100(2), 491 (2017)
D. Zhou, L.-X. Pang, D.-W. Wang, I.M. Reaney, Novel water-assisting low firing MoO3 microwave dielectric ceramics. J. Eur. Ceram. Soc. 39(7), 2374 (2019)
J. Song, G. Zhu, H. Xu, R. Yin, Y. Zhao, X. Zhang, D. Yan, S. Long, T. Wei, Preparation of high-density Bi2O3 ceramics by low temperature sintering. J. Mater. Sci. 31(7), 5214 (2020)
C. Zhong, Y. Deng, W. Hu, J. Qiao, L. Zhang, J. Zhang, A review of electrolyte materials and compositions for electrochemical supercapacitors. Chem. Soc. Rev. 44(21), 7484 (2015)
M.-J. Pan, C.A. Randall, A brief introduction to ceramic capacitors. IEEE Electr. Insulat. Mag. 26(3), 44 (2010)
C.-H. Hong, H.-P. Kim, B.-Y. Choi, H.-S. Han, J.S. Son, C.W. Ahn, W. Jo, Lead-free piezoceramics–where to move on? J. Materiom. 2(1), 1 (2016)
Y. Zhang, Y.-J. Heo, M. Park, S.-J. Park, Recent advances in organic thermoelectric materials: principle mechanisms and emerging carbon-based green energy materials. Polymers 11(1), 167 (2019)
W.D. Kingery, H.K. Bowen, D.R. Uhlmann, Introduction to Ceramics (Wiley, Hoboken, 1976)
X. Zhao, J. Guo, K. Wang, T. Beauvoir, B. Li, C.A. Randall: Introducing a ZnO-PTFE (polymer) nanocomposite varistor via the cold sintering process. Adv. Eng. Mater. https://doi.org/10.1002/adem.201700902 (2018)
E.M. Gutman: Mechanochemistry of solid surfaces (World Scientific Publishing Company, City, 1994)
W. Kingery, J. Woulbroun, F. Charvat, Effects of applied pressure on densification during sintering in the presence of a liquid phase. J. Am. Ceram. Soc. 46(8), 391 (1963)
Y. Cai, B.-M. Zhang Newby, Marangoni flow-induced self-assembly of hexagonal and stripelike nanoparticle patterns. J. Am. Chem. Soc. 130(19), 6076 (2008)
W.B. Hong, L. Li, M. Cao, X.M. Chen, Plastic deformation and effects of water in room-temperature cold sintering of NaCl microwave dielectric ceramics. J. Am. Ceram. Soc. 101(9), 4038 (2018)
H. Kähäri, M. Teirikangas, J. Juuti, H. Jantunen, N. Alford, Dielectric properties of lithium molybdate ceramic fabricated at room temperature. J. Am. Ceram. Soc. 97(11), 3378 (2014)
P. Atkins, J. De Paula, Elements of Physical Chemistry (Oxford University Press, Oxford, 2013)
R. Fernandez-Prini, Le Chatelier’s principle and the prediction of the effect of temperature on solubilities. J. Chem. Educ. 59(7), 550 (1982)
M.M. Renfew: NIOSH Pocket guide to chemical hazards (US Department of Health and Human Services-National Institute for Occupational Safety and Health), (ACS Publications, City, 1991)
G. Taveri, S. Grasso, F. Gucci, J. Toušek, I. Dlouhy, Bio-inspired hydro-pressure consolidation of silica. Adv. Funct. Mater. 28(48), 1805794 (2018)
H. Kähäri, M. Teirikangas, J. Juuti, H. Jantunen, N. Alford, Improvements and modifications to room-temperature fabrication method for dielectric Li2MoO4 ceramics. J. Am. Ceram. Soc. 98(3), 687 (2015)
L. Li, W.B. Hong, S. Yang, H. Yan, X.M. Chen, Effects of water content during cold sintering process of NaCl ceramics. J. Alloys Compd. 787, 352 (2019)
D. Wang, D. Zhou, S. Zhang, Y. Vardaxoglou, W.G. Whittow, D. Cadman, I.M. Reaney, Cold-sintered temperature stable Na0.5Bi0.5MoO4–Li2MoO4 microwave composite ceramics. ACS Sustain. Chem. Eng. 6(2), 2438 (2018)
D. Wang, S. Zhang, D. Zhou, K. Song, A. Feteira, Y. Vardaxoglou, W. Whittow, D. Cadman, I.M. Reaney, Temperature stable cold sintered (Bi0.95Li0.05)(V0.9Mo0.1)O4-Na2Mo2O7 microwave dielectric composites. Materials 12, 9 (2019)
D. Wang, J. Chen, G. Wang, Z. Lu, S. Sun, J. Li, J. Jiang, D. Zhou, K. Song, I.M. Reaney, Cold sintered LiMgPO4 based composites for low temperature co-fired ceramic (LTCC) applications. J. Am. Ceram. Soc. 103, 6237 (2020)
H. Kähäri, M. Teirikangas, J. Juuti, H. Jantunen, Room-temperature fabrication of microwave dielectric Li2MoO4–TiO2 composite ceramics. Ceram. Int. 42(9), 11442 (2016)
I.J. Induja, M.T. Sebastian, Microwave dielectric properties of mineral sillimanite obtained by conventional and cold sintering process. J. Eur. Ceram. Soc. 37(5), 2143 (2017)
M. Väätäjä, H. Kähäri, J. Juuti, H. Jantunen, Li2MoO4-based composite ceramics fabricated from temperature- and atmosphere-sensitive MnZn ferrite at room temperature. J. Am. Ceram. Soc. 100(8), 3626 (2017)
S.S. Faouri, A. Mostaed, J.S. Dean, D. Wang, D.C. Sinclair, S. Zhang, W.G. Whittow, Y. Vardaxoglou, I.M. Reaney, High quality factor cold sintered Li2MoO4-BaFe12O19 composites for microwave applications. Acta Materialia. 166, 202 (2019)
Y. Ji, K. Song, X. Luo, B. Liu, H. Barzegar Bafrooei, D. Wang, Microwave dielectric properties of composite ceramics fabricated by cold sintering process. Front. Mater. 6, 256 (2019)
S.N.A. Rajan, Garnet mineral based composites through cold sintering process: microstructure and dielectric properties. J. Eur. Ceram. Soc. 40(2), 371 (2020)
Y. Ji, K. Song, S. Zhang, Z. Lu, G. Wang, L. Li, D. Zhou, D. Wang and I.M. Reaney: Cold sintered, temperature-stable CaSnSiO5-K2MoO4 composite microwave ceramics and its prototype microstrip patch antenna Journal of the European Ceramic Society. (2020)
W.B. Hong, L. Li, H. Yan, X.M. Chen, Cold sintering and microwave dielectric properties of dense HBO2-II ceramics. J. Am. Ceram. Soc. 102(10), 5934 (2019)
B. Liu, L. Li, K.X. Song, M.M. Mao, Z. Lu, G. Wang, L. Li, D. Wang, D. Zhou, A. Feteira and I.M. Reaney: Enhancement of densification and microwave dielectric properties in LiF ceramics via a cold sintering and post-annealing process. J. Eur. Ceram. Soc. (2020)
H. Kähäri, P. Ramachandran, J. Juuti, H. Jantunen, Room-temperature-densified Li2MoO4 ceramic patch antenna and the effect of humidity. Int. J. Appl. Ceram. Technol. 14(1), 50 (2017)
Acknowledgments
This work was supported by the Engineering and Physical Sciences Research Council (EPSRC) funded through Grant Nos. EP/L017563/1 and EP/N010493/1; Henry Royce Institute for Advanced Materials funded through EPSRC Grants EP/R00661X/1, EP/S019367/1, EP/P02470X/1 and EP/P025285/1.
Author information
Authors and Affiliations
Corresponding authors
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
Open access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, D., Li, L., Jiang, J. et al. Cold sintering of microwave dielectric ceramics and devices. Journal of Materials Research 36, 333–349 (2021). https://doi.org/10.1557/s43578-020-00029-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/s43578-020-00029-w