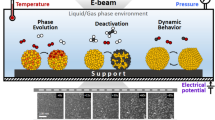
Abstract
Catalysis is a highly complex phenomenon involving fundamental processes on multiple length scales. The full-scale complexity of catalysis is only poorly understood, and how atomic-scale processes influence long-range order in the materials is not well documented experimentally. The result is that we still, to a large degree, develop new catalysts on the basis of iterative trial-and-error approaches. Elucidating the link between atomic-scale structural dynamics, feedback mechanisms, and collective behavior could be the key to a deeper understanding and further optimization of catalysts and processes. From imaging of quasi-static low-energy configurations through gas-phase-induced state switching to observation of complex nonequilibrium dynamics and oscillatory behavior, electron microscopy has provided novel insights over several length and time scales and has meanwhile matured from a service tool for catalyst researchers to a driving force in catalysis research. Here, we discuss new insights provided by novel instrumentation and the extension from in situ to operando investigations, enabling the study of mechanisms and kinetics of catalytic processes.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Heterogeneously catalyzed chemical reactions are complex nonequilibrium processes in which interactions between catalyst, reactants, and products occur on different length and time scales. Due to the lack of atomistic understanding, catalyst optimization has for decades been largely based on an Edisonian approach, where different synthesis protocols and catalyst compositions are systematically tested, often by changing one parameter at a time. One of the most striking examples of this incremental empiricism is the discovery of the first ammonia synthesis catalyst by Mittasch et al. at BASF in 1909, followed by a “systematic exploration of the periodic table,” which involved some 20,000 experiments.1,2 This systematic testing and quasi-empirical optimization laid the foundation for modern high-throughput (HT) parallel screening of catalysts that is still pretty much the state of the art in catalyst development.3,4 Considering the important role of catalysis as a key enabling technology for the realization of a sustainable society, this is a very unsatisfactory situation.
One of the main reasons for our limited understanding of heterogeneous catalysis is rooted in the multiscale nature of the underlying processes. At the nanoscale, the surface properties of the catalyst play a crucial role in determining the reaction mechanism and kinetics, while at the macroscale, transport phenomena such as mass and heat transfer can significantly affect conversion and selectivity. Elementary electronic transitions take place on ultrashort time scales, while atomic motion, species diffusion, heat transport, and restructuring processes, and ultimately, catalyst deactivation, cover the temporal range from being too fast to impractically slow for direct observation. Catalyst development is thus largely based on indirect feedback from measured activity, yield and stability, and pre- and post-characterization and thanks to the development of in situ (observing under specific conditions [i.e., gas environment and temperature in the case of catalysis]) and operando (in situ plus detection of products [i.e., confirmation of reactivity in the case of catalysis]) techniques, on integrally measured structural and compositional information.
When it comes to structural characterization at the nanometer scale, catalyst researchers have often turned to electron microscopy. Scanning electron microscopy provides access to structure, composition, surface topology, and to some extent, particle sizes. Using transmission electron microscopy, researchers can gain insight into atomic arrangement, composition, and electronic structure. Almost half a century ago, Marks and Howie published studies on the shape and structure of supported metal nanoparticles.5 Several decades later, with improved instrumentation and the development of aberration correctors, microscopy experts were able to reveal atomistic details regarding particle shape and surface termination.6,7,8,9 Around the same time, computational chemistry entered the field of catalysis.10 Electronic-structure calculations, mostly in the form of density functional theory (DFT)-based ground-state simulations,11 were used to find descriptors for chemical bonds between a surface and a molecule and to identify trends in reactivity (so-called scaling relations).12,13 Both high-resolution imaging of atomic arrangements in vacuum and DFT-based electronic-structure calculations have contributed to a bias toward a rather static picture of catalysts that has dominated in the field since the concept of the catalytic site was introduced by Taylor in the 1920s.14 Structural features, such as steps or defects, were identified as candidates for high-energy active sites and the determination of molecular processes occurring at the active site became a top priority in catalysis research.15,16 At that time, means of increasing defect density, for example, by high-energy ball milling, were considered a rational approach to the preparation of improved catalysts. Somewhat in contrast, but not incompatible with the static active site picture, were studies on catalyst deactivation, which provided clear evidence that the catalyst material can undergo severe changes and that the actual working state may lie somewhere in between the as-synthesized and the deactivated, postmortem catalyst.17,18 Meanwhile, it is widely accepted that catalysts are metastable functional materials that adapt dynamically to the applied conditions and that relevant surface structures and active centers may form only under reaction conditions.19,20
Since the development and commercialization of instrumentation for in situ and operando EM, we have seen tremendous progress in the capability of addressing the structure of catalysts under reactive environments and associated to that, the role of microscopy in catalysis research. Its impact and potential have been summarized in several reviews over the last decades.21,22,23,24,25,26 In this article, using the example of supported nanoparticles and model catalysts, we highlight the importance of moving beyond structural description of static atomic arrangements and show that the consideration of kinetic and thermodynamic aspects that determine out-of-equilibrium material states and dynamics is essential for our understanding of the emergence of catalytic function. Furthermore, we point out that a multiscale approach to operando electron microscopy facilitates the embedding of locally observed atomistic processes into a larger picture where collective dynamics related to heat and mass flow are revealed.
Observing changes: From static pictures to gas-phase- and temperature-induced processes
The first example presented in Figure 1 shows the case of industrially relevant Cu/ZnO/Al2O3 catalysts for methanol synthesis. The copper-zinc oxide system is recognized as a system in which synergistic particle–support interactions play an important role in defining the function of the catalysts. They result in catalytic performance that far exceeds that of the individual components. However, studies regarding the state of the system under reaction conditions and attempts to identify the active site often led to different, sometimes seemingly contradicting conclusions, which are, 50 years after its first commercial use, still open to discussion. The study by Hansen et al.27 on a model system consisting of copper nanoparticles on a zinc oxide clearly showed adsorbate-induced stabilization of different Wulff-shaped low-energy configurations as previously described by Woodruff.28 They are characterized by different areal ratios between exposed facets and show a clearly noticeable change in wetting behavior (Figure 1a). Images taken by environmental TEM (ETEM) at pressures in the range of 1.5–5 mbar at 220°C under H2, H2 + H2O, and H2 + CO showed that the wetting, and thus the interaction strength between the copper particles and the zinc oxide support, increased with increasing reduction potential of the applied gas phase (Figure 1a). Indeed, reductive activation in a hydrogen/argon mixture (20:80) at 250°C and atmospheric pressure increases the metal–support interaction to the extent that the copper particles become completely encapsulated by a thin zinc oxide layer, as demonstrated by ex situ TEM29 (Figure 1b). The encapsulated state is consistent with an earlier conclusion based on aberration-corrected imaging, according to which the active site consists of Cu steps decorated with Zn atoms (Figure 1c).30 In a rather static picture, it was proposed that these sites are stabilized by well-defined bulk defects and surface species that need to be present together for the system to function. Other studies have suggested that the presence of a copper–zinc alloy is responsible for the high activity, whereas some have proposed a more dynamic picture in which copper, copper–zinc alloy, and zinc oxide phases can coexist.31 Different conclusions, each correct for the respective (nonreactive) regime studied, can be attributed to the fact that the structure and phase composition of the catalyst show a strong pressure dependence, especially below 1 bar (see Figure 1d).32 This example shows that the pressure and thus, the chemical potential of the gas-phase components have a strong influence on the state evolution, thermodynamic stability, and kinetics of a system. Therefore, effects related to the pressure gap between model studies performed at low-pressure and real-world conditions applied in industrial processes should be considered. At elevated pressures, new phases may form that do not exist at lower pressures for thermodynamic reasons. If these phases determine the catalytic activity, then any extrapolation from low- to high-pressure conditions will obviously fail.32 Nevertheless, even if conclusions drawn on observations of a system at low pressure can not directly be transferred to high-pressure regimes, there is no doubt that the description of static, quasi-equilibrium low-energy states (i.e., states that are very close to thermodynamic equilibrium) is important. They could, for example, play an important role as beginning or end point of an elementary step in a catalytic cycle. However, studies performed under quasi-equilibrium conditions insufficiently describe the behavior of catalysts under nonequilibrium conditions of an ongoing chemical reaction. Indeed, the previously discussed studies do not provide information about the structural and chemical dynamics under working conditions. Questions regarding the atomic-scale evolution and aspects of the metal-support interaction remain open. For example, how does the metal particle and the metal–support interface behave during catalysis if oxygen vacancies are constantly being created and annihilated? This will be discussed in the following examples.
(a) Cu particles on a ZnO support show gas-phase-induced adaption of Wulff-shaped minimum energy configurations under quasi-equilibrium conditions. Reproduced with permission from Reference 27. © 2002 AAAS. (b) Copper particles showing strong metal–support interaction-induced encapsulation by a layered form of ZnO after reductive activation. Reprinted with permission from Reference 29. © 2015 Wiley-VCH. KGaA, Weinheim. (c) High-resolution images of Cu on ZnO and structure model showing surface features associated to active sites. Reprinted with permission from Reference 30. © 2012 AAAS. (d) Schematic representation of the evolution of the phase composition during reductive treatment of a Cu–ZnO–Al2O3 precursor as a function of temperature and pressure. Reprinted with permission from Reference 32. © 2021 Springer Nature.
Observing dynamics: From quasi-equilibrium states to nonequilibrium processes
Staying with the topic of metal–support interactions, we move from descriptions of quasi-equilibrium states to gas-phase and electron-beam-induced state switching between different low-energy configurations. One example is the study of Yuan et al., who reported a gasphase-induced reversible in-plane (epitaxial) rotation behavior of gold particles supported on a TiO2 surface (see Figure 2a). A perfect epitaxial Au/TiO2(001) interface (S″ configuration), Au(111)//TiO2(001) with zone axes Au[01–1] and TiO2[010] was found in an oxygen environment (6.5 mbar, 500°C). As carbon monoxide was added to the oxygen stream (total pressure: 4.4 mbar, pO2:pCO 1:2, 500°C), a reproducible rotation of the gold nanoparticles with respect to the substrate was observed (S = configuration).33 The mechanism behind the rotation was ascribed to a decrease of the oxygen coverage at the perimeter sites at the interface between gold and titania upon introduction of CO. In consequence, the epitaxial relationship that is most favorable in oxygen reversibly changed to a new, slightly rotated low-energy configuration. This conclusion was supported by density functional theory-based calculations on smaller systems showing how the coverage of adsorbed oxygen at the perimeter sites influence the epitaxial relationship between nanoparticle and support.
(a) Gas-phase-induced reversible in-plane (epitaxial) rotation behavior of Au nanoparticles on TiO2. Reprinted with permission from Reference 33. © 2021 AAAS. (b) Reversible rigid body displacement of Au nanoparticles on a CeO2 support. Reprinted with permission from Reference 34. © 2013 American Chemical Society. (c) Sequential disappearance of TiOx overlayers from different surface facets of Pt particles with increasing hydrogen partial pressure. Reprinted with permission from Reference 35. © 2022 AAAS.
A similar study on dynamic catalytic interfaces was presented by Kuwauchi et al. for the case of gold nanoparticles on a cerium oxide support.34 They observed a reversible rigid body displacement, characterized by stepwise back and forth movement or rotation around an anchor point (see Figure 2b). In their study, the displacement was induced when the system was exposed to reaction environments containing oxidizing and reducing species (100 Pa of 1 vol% CO/air at room temperature). Probably related to the low chemical potential of CO (low partial pressure and low temperature), the electron beam was needed to assist in the generation of oxygen vacancies at the interface between metal particle and reducible support. The reversibility of the observed displacement was related to the refilling of oxygen vacancies at the interface through oxygen from the gas phase. A corresponding increase in the frequency of the stepwise displacement was observed when the chemical potential of oxygen was increased.
These two examples describe the state switching of a system between two different low-energy configurations. In both cases, the reconfiguration at the interface between metal particle and reducible support was induced through (assisted) oxygen removal by CO and its replenishment.
Interfacial destabilization and reconfiguration can be more severe at higher chemical potentials of the reactive species. Working at pressures of 1 bar, Frey et al. recently demonstrated that metal–support interfaces can be significantly destabilized by redox processes.35 Starting with strong metal–support interaction (SMSI)-encapsulated platinum particles on titania, the switching from a pure oxygen atmosphere to a redox atmosphere containing oxygen and hydrogen resulted in a gradual destabilization of the thin titania overlayer. Figure 2c shows the sequential disappearance of the titania overlayer from different surface facets with increasing hydrogen partial pressure. The sequential overlayer disintegration indicates a facet-dependent stability of differently structured SMSI overlayers on platinum. Its ultimate disappearance suggests that the SMSI state, which has been shown to provide tunable chemoselectivity36 and improved sintering resistance,37 is not necessarily preserved under reaction conditions. Indeed, the degree of particle encapsulation can vary greatly depending on the conditions applied, as was already shown by Hansen et al. for the case of a Ba-promoted Ru catalyst.38
Redox processes that destabilize the thin encapsulating titania layer on the platinum particles can furthermore destabilize the remaining platinum titania interface (i.e., the one between the particle and the support). Thus, once the surface of the de-encapsulated platinum particle is exposed to the redox atmosphere, efficient hydrogen activation leads to migration (spillover) of hydrogen atoms toward the Pt/TiO2 interface. Strain modulation at the heteroepitaxial interface facilitates vacancy generation.39 Aggregation of oxygen vacancies in titania results in so-called Wadsley defects.40,41,42,43,44 The associated interface reconstruction and formation of shear planes induce a morphological adaptation of the supported particle. With increasing extent of reduction, the local affinity of TiOx toward reoxidation increases up to the point where reoxidation via gas-phase oxygen can take place. This again induces a morphological adaptation of the Pt nanoparticles. Depending on the relative orientation of the platinum nanoparticles and the exposed titania surface, the ongoing Mars–van Krevelen-like redox processes45 at the Pt–TiO2 interface can give rise to different structural dynamics of the supported particles. Three cases, including one that results in directed particle migration, are exemplified in the article by Frey et al. and shown in Figure 3a. While in the previous examples, due to observation at low pressure and associated low conversion rate, no product formation and thus catalytic activity could be detected, a correlation between the observed particle dynamics and catalytic water formation could be found in the study carried out at 1 bar by Frey et al. Experiments conducted at different H2/O2 ratios and under consideration of the effect of water vapor, furthermore showed that dynamics occur as long as the conditions are neither too reducing nor too oxidizing with respect to the chemical stability of the different configurations between which the system can switch (i.e., states with reduced and reoxidized Pt/TiO2 interfaces).
(a) Structural dynamics of differently oriented Pt particles on titania under reactive conditions. Depending on the relative orientation, different dynamics related to twinning and step flow on Pt 111 planes are observed. Reprinted with permission from Reference 35 © 2022 AAAS. (b) Pt nanoparticles on ceria in vacuum and under reactive conditions, where fast restructuring leads to a blurring of lattice fringes. With increasing chemical potential of CO, turnover frequency and dynamics increase. Reprinted with permission from Reference 46. © 2021 Springer Nature.
Crozier et al. arrived at a similar conclusion regarding the cause of the atomic-level fluxional behavior of nanometer-sized platinum particles on ceria under CO oxidation conditions (see Figure 3b).46 Based on an earlier study,47 they were able to relate the fluxional behavior of destabilized platinum particles to the restructuring of the ceria surface via Mars–van Krevelen reduction and reoxidation processes. They were furthermore able to show that the catalytic turnover frequency correlates with the fluxional behavior through the associated rate of oxygen vacancy creation and annihilation.46 This finding is important, because it implies that the detection of chemical conversion is unlikely in static systems. This work also provides direct insight into the adaptive behavior of a catalyst and reveals fluxionality, reconstruction, reshaping, and dynamic structural transformations that can only occur under nonequilibrium conditions. Indeed, if the chemical potential of the reactive species in the gas phase is sufficient (i.e., if the driving force is high enough with respect to the chemical stability of a given catalyst configuration), then changes in surface termination48,49 particle shape,50 interface configuration,35,46 state of encapsulation or internal structure51 can be induced. If the reactants of the gas phase differ in their effect on the chemical state of the catalyst, for example, in a redox atmosphere and if the chemical forces are balanced (i.e., none of the reactants dominates and leads to the stabilization of a particular state), then continuous state switching can be induced. Under such conditions, the system behaves as a chemically driven oscillator in which reaction cycles are coupled to dynamical processes.50,52,53
State switching has an embedded mechanism in which active centers can be periodically regenerated. Under continuous gas flow and temperature exchange, the thermodynamically open dissipative system52 remains in a chemically driven nonequilibrium state. Interruption of the gas flow and temperature change drives the system out of this dynamic regime and toward an equilibrium state.
From local atomistic dynamics to collective behavior
So far, we have considered local observation of atomistic processes. Some processes, such as the spillover of hydrogen previously mentioned, can affect regions beyond the local particle support interface. Using lithographically deposited platinum structures on a ceria support, Beck et al.54 have shown that spillover of activated hydrogen from platinum onto cerium dioxide can lead to propagating reduction fronts. Depending on the applied conditions, these reduction fronts can extend several microns from the activation site. Propagating reaction fronts are one of many coupling phenomena that determine the macroscopic behavior of a reaction. Ertl famously observed propagating reaction fronts using photoemission electron microscopy (PEEM) during CO oxidation on platinum single crystals (Figure 4a)55 and studied oscillatory kinetics and spatiotemporal self-organization in reactions on solid surfaces.56 Meanwhile, there have been many reports on oscillatory kinetics57,58,59 and reactions that involve propagating reaction fronts and formation of dissipative structures60,61 (Figure 4b). The formation of spatiotemporal pattern is typical for nonlinear systems that are operated far from equilibrium62 and has been observed in many reaction systems and natural processes.63 In electrochemistry, for example, almost all reactions show oscillatory behavior within a certain range of external parameters.64 The kinetic mechanisms responsible for oscillatory dynamics and formation of spatiotemporal structures are linked to feedback loops and coupling between processes that occur on different levels in a heterogeneous catalytic reaction. Examples for nonlinear coupling are coverage-dependent adsorption energies, threshold behavior in restructuring and initiation of phase transitions, reaction-induced temperature changes, concentration gradients, and diffusion processes. They all can be involved in feedback loops that lead to oscillatory behavior.52,65 Terms such as “catalytic turnover frequency” and the sequence of adsorption-reaction-desorption that defines a catalytic cycle indicate that catalytic function is inherently linked to oscillatory behavior. Like the above-described fluxional atomic-scale dynamics and state switching, the larger-scale oscillatory dynamics in reaction–diffusion systems can be key in preventing the system from reaching a stable (inactive) equilibrium state and provide a larger-scale mechanism for cyclic regeneration of active centers. They are also linked to preferential adsorption, reaction, and desorption of species in a repetitive reaction sequence.
(a) If undisturbed, lateral coupling of oscillatory dynamics can give rise to propagating reaction fronts and formation of dissipative structures, such as observed by photoemission electron microscopy (PEEM) during CO oxidation on Pt single crystals.56 Correlative electron microscopy enabled through the combination of energy-resolved x-ray PEEM (XPEEM) and low-energy electron microscopy (LEEM) provides additional information about lateral and temporal changes in surface chemical states, for example, during H2 oxidation on Rh. Reprinted with permission from Reference 69. © 2022 American Chemical Society. More locally, field ion microscopy performed on sharp metal tips reveals fluctuating changes in surface coverage and can reveal differences in reactivity between different exposed crystallographic facets. Reprinted with permission from Reference 59. © 2010 American Chemical Society. (b) Propagating reaction fronts observed during NO2 hydrogenation on a polycrystalline Pt foil and a grain orientation map recorded by electron backscatter diffraction. The ability to detect coverage-dependent changes in work function via secondary electron imaging and observation of grain orientation-dependent surface reactivity was demonstrated for the case of NO2 hydrogenation on Pt performed in an environmental scanning electron microscope (ESEM). Reprinted with permission from Reference 61. © 2019 Springer Nature.
To understand the kinetics that determine the function of a catalyst, coupling between gas-phase composition, temperature, and state of the catalyst thus need to be investigated on length scales spanning from local atomic motion,66,67,68 all the way to large-scale collective dynamics.61,69 Without a multiscale approach and by just focusing on atomistic details and linear extrapolation, catalysis cannot be fully understood.
Figure 5 shows an example of a multiscale observation for a simple model system of nickel in hydrogen oxidation. Investigation of individual Ni particles by operando TEM under constant flow of hydrogen and oxygen revealed an oscillatory behavior in which nickel particles periodically switched between the active metallic state and the inactive oxidized form (Figure 5, top row). It is known that kinetic oscillation can be a result of the interplay of rapid chemical reaction steps and relatively slow complementary processes such as adsorbate-induced surface reconstruction or oxide formation.70 During the operando TEM observation, the behavior of individual particles on the MEMS chip was not synchronized and no oscillation in the integrally collected catalytic water formation was observed (not shown).
(a) Oscillatory redox behavior of a Ni particle observed by operando transmission electron microscopy imaging during H2 oxidation in a 20% H2 + 5% O2 + 75% He mixture at 200 mbar. Microelectromechanical chip was heated to 700°C with constant heating power (i.e., thermal oscillation not suppressed). (b) Operando scanning electron microscopy observation of a polycrystalline Ni-foil in 0.3 mbar under constant flow of H2 (85%) and O2 (15%) at 600°C. Simultaneously measured gas-phase composition by mass spectroscopy shows oscillatory (redox and catalytic) behavior of the whole polycrystalline foil. Active (metallic) state is appearing dark in the secondary electron image, while the inactive (oxidized) state shows a higher brightness. (c) When the temperature is fixed, thermokinetic collective oscillation is suppressed and formation of propagating waves corresponding to a reduced (dark) and oxidized (bright) Ni surface is observed.
To understand the reason for the oscillatory phase change and behavior on a larger scale, operando SEM experiments were performed. Real-time imaging under reactive conditions showed that the extended surface of a polycrystalline nickel foil shows similar oscillatory redox behavior as individual particles. Water formation, detected by on-line mass spectrometry, revealed a varying catalytic activity of the surface, characterized by a highly active metallic state and an oxidized surface showing low activity. These collective oscillations between the metallic and oxidized state involved the whole polycrystalline nickel foil (except some less oxygen affine (111)-oriented Ni grains) and are similar to the oscillatory behavior of nickel observed during methane or propane oxidation.71,72 Due to the exothermal water formation in the active phase, the temperature varied in accordance with the oscillation in catalytic activity. The temperature was thus initially considered to play a key role in the feedback mechanism, such as in thermokinetic oscillation. Indeed, when the temperature of the Ni foil was fixed in another experiment via a PID controller connected to the heater, collective thermokinetic oscillation was prevented, and the mass spectroscopy signal indicated a stable production of water. Thanks to operando microscopy, we were able to reveal that the system was still oscillating, but in a different style. What seemed to be a steady state with constant catalytic conversion, based on integral mass spectrometry of the gas-phase composition in the ESEM chamber, was in fact the product of coexisting active and inactive domains on the surface. Real-time imaging of the active surface under conditions of fixed temperature revealed propagating reaction fronts separating oxidized and reduced areas of the nickel catalyst. These propagating waves act as chemical clocks and influence the reaction dynamics on differently oriented grains by cross-coupling. Local dynamics are thus influenced through long-range effects of traveling waves. This example of the behavior of nickel in the most elementary redox reaction shows that nonlinear dynamics and coupling phenomena can give rise to complex spatiotemporal dynamics even in relatively simple systems. They can be studied conveniently at intermediate magnification but may be overlooked or completely inaccessible if experiments are focused on atomic-scale observation alone.
Conclusion
In situ and operando electron microscopy has contributed substantially to our understanding of catalysis. It has helped to overcome simplified pictures of static active sites and made it widely understood that observation of isolated systems in vacuum is insufficient and can lead to misleading conclusions concerning the functional state of active catalysts.
The dynamic fluxionality and oscillatory behavior observed in the examples discussed here suggest that the active state of a catalyst involves a collection of many structures that dynamically interconvert for as long as the catalyst remains active. Coupling between fast catalytic cycles (adsorption, reaction, desorption) and relatively slow “side” processes, induced by secondary reactions such as adsorbate-induced surface restructuring, substrate reduction and reoxidation, or phase transformation need to be considered in the description of a working catalyst, especially, if they are interlinked and nonseparable from a working state.
The combination of in situ and operando electron microscopy provides important insight into the sensitive balance between the processes playing hand-in-hand at different temporal and lateral scales. On one hand, observation of static atomic configurations under quasi-equilibrium conditions is essential because it delivers detailed information about low-energy states between, which the active system is eventually oscillating under reactive nonequilibrium conditions. On the other hand, observation under operando conditions is essential, because it is only by studying function that we can understand function. Here, the system is operating beyond the quasi-static regime and the speed of atomic motion can be rather fast. Short exposure times and the need to keep the electron dose rate as low as possible set limits with regard to what can still be imaged. Indeed, electron-beam-induced artifacts are often encountered in electron microscopy and should be avoided or understood, because they can influence the reaction mechanism and kinetics.25 If atomistic dynamics get too fast to follow, it makes sense to consider observation at lower magnification, where the focus is shifting toward the study of collective and nonlinear dynamics and where electron dose limitations are partially circumvented due to observation at reduced magnification.
The aim of this article was to point out that the study of complex multiscale phenomena, such as heterogeneous catalysis, requires a toolset that can capture relevant working states at different lateral and temporal scales. The combination of in situ and operando SEM and TEM allows us to gain novel insight from the atomic level to lateral dimensions up to microns and provides important insights into mechanisms and kinetics of catalytic processes. The consideration of a multiscale approach and expansion of the focus from high-resolution TEM also toward SEM and complementary electron microscopy-based tools such as in situ photoemission and field ion microscopy seems viable and will foster the investigation of effects related to pressure and material gaps in systematic ways. Electron microscopy is in a unique position in catalysis research and has already changed from a simple characterization tool that is operated as service tool for the catalyst researcher in academic environments to a method that is driving the field forward and is recognized by industry. It helps to overcome the shortcomings of semiempirical trial-and-error based progress and provides new and relevant insights into the working state of active catalysts.
Code availability
Not applicable.
References
A. Kreimeyer, P. Eckes, C. Fischer, H. Lauke, P. Schuhmacher, Angew. Chem. Int. Ed. Engl. 54, 3178 (2015)
D. Farrusseng, Surf. Sci. Rep. 63, 487 (2008)
R.J. Hendershot, C.M. Snively, J. Lauterbach, Chemistry 11, 806 (2005)
J.A. Moulijn, J. Perez-Ramirez, R.J. Berger, G. Hamminga, G. Mul, F. Kapteijn, Catal. Today 81, 457 (2003)
L.D. Marks, A. Howie, Nature 282, 196 (1979)
B. Freitag, S. Kujawa, P.M. Mul, J. Ringnalda, P.C. Tiemeijer, Ultramicroscopy 102, 209 (2005)
L.D. Marks, D.J. Smith, Nature 303, 316 (1983)
W.Z. Zhou, J.M. Thomas, Curr. Opin. Solid State Mater. Sci. 5, 75 (2001)
J.Y. Liu, Microsc. Microanal. 10, 55 (2004)
J. Greeley, J.K. Norskov, M. Mavrikakis, Annu. Rev. Phys. Chem. 53, 319 (2002)
W. Kohn, A.D. Becke, R.G. Parr, J. Phys. Chem. 100, 12974 (1996)
J.K. Nørskov, F. Abild-Pedersen, F. Studt, T. Bligaard, Proc. Natl. Acad. Sci. U.S.A. 108, 937 (2011)
G. Jones, T. Bligaard, F. Abild-Pedersen, J.K. Norskov, J. Phys. Condens. Matter 20, 6 (2008)
H.S. Taylor, Proc. R. Soc. London Ser. A Contain. Papers Math. Phys. Character 108, 105 (1925)
A.P. Amrute, J. De Bellis, M. Felderhoff, F. Schuth, Chem. Eur. J. 27, 6819 (2021)
I. Ayub, D.S. Su, M. Willinger, A. Kharlamov, L. Ushkalov, V.A. Zazhigalov, N. Kirillova, R. Schlogl, Phys. Chem. Chem. Phys. 5, 970 (2003)
J. Sehested, A. Carlsson, T. Janssens, P. Hansen, A. Datye, J. Catal. 197, 200 (2001)
A.K. Datye, D.J. Smith, Catal. Rev. Sci. Eng. 34, 129 (1992)
G. Ertl, Top. Catal. 1, 305 (1994)
R. Schlogl, Angew. Chem. Int. Ed. 54, 3465 (2015)
P.L. Gai, Top. Catal. 8, 97 (1999)
P.L. Hansen, S. Helveg, A.K. Datye, Adv. Catal. 50, 77 (2006)
P.A. Crozier, T.W. Hansen, MRS Bull. 40(1), 38 (2015)
R.T.K. Baker, Catal. Rev. Sci. Eng. 19(2), 161 (1979)
S.W. Chee, T. Lunkenbein, R. Schlogl, B.R. Cuenya, J. Phys. Condens. Matter 33, 28 (2021)
P.L. Gai, Top. Catal. 21, 161 (2002)
P.L. Hansen, J.B. Wagner, S. Helveg, J.R. Rostrup-Nielsen, B.S. Clausen, H. Topsøe, Science 295, 2053 (2002)
D.P. Woodruff, J. Phys. Condens. Matter 6, 6067 (1994)
T. Lunkenbein, J. Schumann, M. Behrens, R. Schlogl, M.G. Willinger, Angew. Chem. Int. Ed. 54(15), 4544 (2015)
M. Behrens, F. Studt, I. Kasatkin, S. Kühl, M. Hävecker, F. Abild-Pedersen, S. Zander, F. Girgsdies, P. Kurr, B.-L. Kniep, M. Tovar, R.W. Fischer, J.K. Nørskov, R. Schlögl, Science 336, 893 (2012)
N.Y. Topsøe, H. Topsøe, Top. Catal. 8, 267 (1999)
A. Beck, M. Zabilskiy, M.A. Newton, O. Safonova, M.G. Willinger, J.A. van Bokhoven, Nat. Catal. 4, 488 (2021)
W. Yuan, B. Zhu, K. Fang, X.-Y. Li, T.W. Hansen, Y. Ou, H. Yang, J.B. Wagner, Y. Gao, Y. Wang, Z. Zhang, Science 371, 517 (2021)
Y. Kuwauchi, S. Takeda, H. Yoshida, K. Sun, M. Haruta, H. Kohno, Nano Lett. 13(7), 3073 (2013)
H. Frey, A. Beck, X. Huang, J.A. van Bokhoven, M.G. Willinger, Science 376, 983 (2022)
Z. Luo, G. Zhao, H. Pan, W. Sun, Adv. Energy Mater. 12, 2201395 (2022)
H.L. Tang, Y. Su, B.S. Zhang, A.F. Lee, M.A. Isaacs, K. Wilson, L. Li, Y.G. Ren, J.H. Huang, M. Haruta, B.T. Qiao, X. Liu, C.Z. Jin, D.S. Su, J.H. Wang, T. Zhang, Sci. Adv. 3, 8 (2017)
T.W. Hansen, J.B. Wagner, P.L. Hansen, S. Dahl, H. Topsøe, C.J.H. Jacobsen, Science 294, 1508 (2001)
A.R. Puigdollers, P. Schlexer, S. Tosoni, G. Pacchioni, ACS Catal. 7, 6493 (2017)
C. Bäumer, R. Dittmann, “Redox-Based Memristive Metal-Oxide Devices,” in Metal Oxide-Based Thin Film Structures (Elsevier, 2018), chap. 20, p. 489
R.J. Kamaladasa, A.A. Sharma, Y.T. Lai, W.H. Chen, P.A. Salvador, J.A. Bain, M. Skowronski, Y.N. Picard, Microsc. Microanal. 21, 140 (2015)
D.S. Jeong, H. Schroeder, U. Breuer, R. Waser, J. Appl. Phys. 104, 8 (2008)
L.A. Bursill, B.G. Hyde, Philos. Mag. 23, 3 (1971)
L.A. Bursill, B.G. Hyde, D.K. Philp, Philos. Mag. J. Theor. Exp. Appl. Phys. 23(186), 1501 (1971)
P. Mars, D.W. van Krevelen, Chem. Eng. Sci. 3, 41 (1954)
J.L. Vincent, P.A. Crozier, Nat. Commun. 12, 5789 (2021)
P.A. Crozier, R. Wang, R. Sharma, Ultramicroscopy 108, 1432 (2008)
R. Imbihl, M.P. Cox, G. Ertl, J. Chem. Phys. 84, 3519 (1986)
B.K. Miller, P.A. Crozier, ACS Catal. 11, 1456 (2021)
S.B. Vendelbo, C.F. Elkjaer, H. Falsig, I. Puspitasari, P. Dona, L. Mele, B. Morana, B.J. Nelissen, R. van Rijn, J.F. Creemer, P.J. Kooyman, S. Helveg, Nat. Mater. 13, 884 (2014)
C. Vogt, F. Meirer, M. Monai, E. Groeneveld, D. Ferri, R.A. van Santen, M. Nachtegaal, R.R. Unocic, A.I. Frenkel, B.M. Weckhuysen, Nat. Commun. 12, 7096 (2021)
R. Imbihl, G. Ertl, Chem. Rev. 95, 697 (1995)
S. Amano, S. Borsley, D.A. Leigh, Z.H. Sun, Nat. Nanotechnol. 16, 1057 (2021)
A. Beck, D. Kazazis, Y. Ekinci, X. Li, E.A. Muller Gubler, A. Kleibert, M.G. Willinger, L. Artiglia, J.A. van Bokhoven, ACS Nano 17, 1091 (2022)
S. Nettesheim, A. von Oertzen, H.H. Rotermund, G. Ertl, J. Chem. Phys. 98, 9977 (1993)
G. Ertl, Science 254, 1750 (1991)
F. Schuth, B.E. Henry, L.D. Schmidt, Adv. Catal. 39(39), 51 (1993)
Z. Kurtanjek, M. Sheintuch, D. Luss, J. Catal. 66, 11 (1980)
J.S. Mcewen, P. Gaspard, Y. De Decker, C. Barroo, T. Visart, Langmuir 26, 16381 (2010)
P. Winkler, J. Zeininger, M. Raab, Y. Suchorski, A. Steiger-Thirsfeld, M. Stoger-Pollach, M. Amati, L. Gregoratti, H. Gronbeck, G. Rupprechter, Nat. Commun. 12, 8 (2021)
C. Barroo, Z.J. Wang, R. Schloegl, M.G. Willinger, Nat. Catal. 3, 30 (2020)
G. Nicolis, I. Prigogine, Proc. Natl. Acad. Sci. U.S.A. 68, 2102 (1971)
G.M. Whitesides, B. Grzybowski, Science 295, 2418 (2002)
K. Krischer, N. Mazouz, P. Grauel, Angew. Chem. Int. Ed. 40, 851 (2001)
U.F. Franck, Angew. Chem. Int. Ed. Engl. 17(1), 1 (1978)
C. Barroo, Y. De Decker, T.V. de Bocarme, P. Gaspard, J. Phys. Chem. Lett. 6, 2189 (2015)
C. Barroo, Y. De Decker, Phys. Rev. Lett. 117, 144501 (2016)
J. Zeininger, M. Raab, Y. Suchorski, S. Buhr, M. Stöger-Pollach, J. Bernardi, G. Rupprechter, ACS Catal. 12, 12774 (2022)
J. Zeininger, P. Winkler, M. Raab, Y. Suchorski, M.J. Prieto, L.C. Tănase, L. de Souza Caldas, A. Tiwari, T. Schmidt, M. Stöger-Pollach, A. Steiger-Thirsfeld, B. Roldan Cuenya, G. Rupprechter, ACS Catal. 12, 11974 (2022)
M.M. Slinko, Catal. Today 154, 38 (2010)
M.M. Slinko, V.N. Korchak, N.V. Peskov, Appl. Catal. A 303, 258 (2006)
V.V. Kaichev, D. Teschner, A.A. Saraev, S.S. Kosolobov, A.Y. Gladky, I.P. Prosvirin, N.A. Rudina, A.B. Ayupov, R. Blume, M. Havecker, A. Knop-Gericke, R. Schlogl, A.V. Latyshev, V.I. Bukhtiyarov, J. Catal. 334, 23 (2016)
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher′s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hansen, T.W., Willinger, M. From atomistic to collective dynamics: Bridging gaps in gas-phase electron microscopy for catalysis. MRS Bulletin 48, 842–851 (2023). https://doi.org/10.1557/s43577-023-00596-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/s43577-023-00596-3