Abstract
Detailed studies of interfacial gas-phase chemical reactions are important for understanding factors that control materials synthesis and environmental conditions that govern materials performance and degradation. Out of the many materials characterization methods that are available for interpreting gas–solid reaction processes, in situ and operando transmission electron microscopy (TEM) is perhaps the most versatile, multimodal materials characterization technique. It has successfully been utilized to study interfacial gas–solid interactions under a wide range of environmental conditions, such as gas composition, humidity, pressure, and temperature. This stems from decades of R&D that permit controlled gas delivery and the ability to maintain a gaseous environment directly within the TEM column itself or through specialized side-entry gas-cell holders. Combined with capabilities for real-time, high spatial resolution imaging, electron diffraction and spectroscopy, dynamic structural and chemical changes can be investigated to determine fundamental reaction mechanisms and kinetics that occur at site-specific interfaces. This issue of MRS Bulletin covers research in this field ranging from technique development to the utilization of gas-phase microscopy methods that have been used to develop an improved understanding of multilength-scaled processes incurred during materials synthesis, catalytic reactions, and environmental exposure effects on materials properties.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
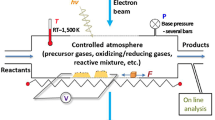
Gas–solid interactions are fundamental to a wide range of technologically important processes, including materials synthesis, surface modification, storage, transport, sensing, and catalysis. It is crucial to study these interactions because when materials are exposed to gaseous environments, their properties, reactivity, and performance can be influenced significantly. The transmission electron microscope (TEM), invented in the late 1930s, has opened new possibilities for exploring dynamic gas-phase processes in real time and at high spatial resolution. However, a major challenge arose due to the requirement of imaging with electrons in a high vacuum environment, which is needed to preserve electron wave coherence, resolution, and to prevent contamination. Fortunately, early pioneers in electron microscopy recognized this and devised innovative approaches to circumvent these issues and developed methods to isolate specific regions of the TEM that would still allow for the maintenance of vacuum conditions while creating controlled regions surrounding the pole piece of the TEM where gas can be introduced and maintained.
To perform gas-phase experiments, two general approaches have been developed that allow for the creation of a controlled gaseous environment within the TEM column or within closed-form gas-cell holders. The first approach, that later led to the development and commercialization of the modern environmental TEM (E-TEM), used small apertures placed above and below the sample to maintain the vacuum even when gas is delivered to the microscope.1 Improvements made over time led to the incorporation of the differentially pumped aperture system that helped to increase the range of allowable pressures within the pole piece of the microscope.2 These advances allowed for the electron beam to pass through the specimen for imaging and analysis unperturbed, allowing studies of dynamic gas–solid chemical reactions to be performed. It is worth noting here that changing the gas pressure in the microscope will have an effect on spatial resolution; however, more recent E-TEMs have been equipped with high brightness field-emission sources and aberration correctors on both the probe- and image-forming lenses for increased spatial resolution and to produce directly interpretable S/TEM images. Concurrent with solving the challenge of creating a controlled environment in the TEM and improving spatial resolution, heating holders were developed and can be used in these instruments to further expand capabilities for studying dynamic processes in a gaseous environment as a function of temperature. The second approach that has been developed uses the concept of “windowed” gas cells. In this case, a controlled gas environment is maintained between paired electron transparent membranes. Silicon fabrication techniques form the basis of fabricating the gas cells, which are essentially silicon microchip devices that support thin, electron transparent SiNx membranes. Thin-film ceramic or metal heating elements are microfabricated onto one of the microchip devices to control temperature.3,4,5 The gas cell is incorporated into the tip of a specially designed TEM holder with integrated tubing and the gas is controllably delivered to the cell through a gas manifold system. This system offers more flexibility than the E-TEM because pressure within the cell can be varied from vacuum to super-atmospheric pressure, and the system can be used in any conventional S/TEM. For more details regarding the evolution of the differentially pumped aperture system that is at the core of the E-TEM and the development of microfabricated gas cells, readers are directed to the following book edited by T.W. Hansen and J.B. Wagner and references therein.6
Articles in this issue
These combined advancements enable the study of dynamic gas–solid interactions under realistic environmental conditions. There have been significant advances in the science that gas-phase microscopy research has enabled. While there have been past review articles, we wanted to update the scientific community on recent developments and applications of gas-phase microscopy because this field is constantly evolving. In this issue of MRS Bulletin, we have invited experts in the field to review the latest developments and application of gas-phase microscopy techniques, with a specific emphasis on technique development and applications to materials synthesis, catalysis, and materials degradation.
Understanding key physical processes in materials synthesis can lead to the design and discovery of new materials. For example, bulk top-down and bottom-up physical vapor deposition systems such as molecular beam epitaxy (MBE) and chemical vapor deposition (CVD), often rely upon and exploit vapor–liquid–solid (VLS) and vapor–solid–solid (VSS) growth mechanisms to synthesize new functional nanomaterials. New insight into how processing parameters influence growth, morphology, and composition can be directly attained using the E-TEM, wherein precursor gases are controllably delivered into the column of the TEM and maintained by differentially pumped apertures in the pole piece surrounding the sample, while the temperature can be controlled by heating the specimen using furnace type or MEMS-based heating holders. A wide range of materials synthesis conditions of gas composition, temperature, and pressure can be explored to synthesize materials within the microscope itself, which can be further correlated with physical property measurements. The E-TEM’s open-cell configuration lends itself exceptionally well for high spatial resolution imaging such that atomic-scale insight into the nucleation and growth mechanisms are readily interpretable. Several main research themes have emerged on how metal catalyst nanoparticles mediate mass transport from the gas-phase precursors and contribute to growth of materials such as carbon nanotubes and semiconductor nanowires.7,8,9,10 Figure 1 highlights a recent E-TEM study in understanding nanomaterial growth mechanisms of GaAs nanowires as a function of droplet contact angle.11 The article by Dick12 in this issue discusses the utilization of E-TEM to understand key nanomaterial growth mechanisms and provides insight into the potential for innovative E-TEM experiments aimed at exploring heat and mass transport-related phenomena in gas-phase materials synthesis.12
Dynamic observations revealing contact angle effects on the self-catalyzed vapor–liquid–solid growth behavior of GaAs nanowires at 420°C. Scale bars = 5 nm. High spatial resolution imaging capabilities within the environmental-transmission electron microscope experiments permit improved insight into the monolayer growth mechanisms that lead to formation of the zinc-blende (ZB) versus wurtzite (WZ) phase. Reprinted with permission from Reference 11. © 2020 American Chemical Society.
In situ and operando S/TEM has also become an indispensable characterization tool in catalysis research to understand the performance of catalysts under their active working environment. Desirable properties such as high activity, selectivity, and durability are the main goals of catalysis research, but from a fundamental point of view, it is the holistic understanding of reaction mechanisms and kinetics occurring at site-specific catalytically active sites that are extremely important to ascertain. Establishing critical links between microstructural features such as catalyst/support interactions, crystallographic facet orientation and strain, with adsorption energy barriers and reaction pathways is needed. E-TEM research has shown how supported noble metal catalyst nanoparticles can restructure in the presence of gas molecules and under reactive conditions with unprecedented atomic detail. Notable examples include the direct imaging of Au nanoparticles supported on CeO2, where it has been shown that the {100} surface of Au nanoparticles undergoes reconstruction in the presence of adsorbed CO molecules.13 Catalyst/support interactions also have important implications in heterogeneous catalysis whereby epitaxial rotation of Au nanoparticles on TiO2 occurs as a function of different mixtures of O2 and CO/O2, which suggests a pathway to tune the interface during catalytic reactions.14 Catalysis research has also benefited from the development of in situ gas-cell holders where it is possible to span a wider range of pressures, even above atmospheric pressure, and temperatures in excess of 1000°C using MEMS-based closed gas cell systems.15 Using these systems, it was uncovered how Pt16 and Pd17 nanoparticle catalysts change shape during CO oxidation reactions and how the surface reconstruction of Pt3Co nanoparticles occurs under oxidation conditions.18 A representative example of gas interactions during catalytic reactions occuring at the catalyst interface is shown in Figure 2. The article by Willinger and Hansen in this issue provides several comprehensive case studies that reveal the atomic scale to collective dynamics of catalytic reactions as imaged and analyzed through in situ/operando experiments in both S/TEM and scanning electron microscopy (SEM) instruments.19 Combining experimental observations with first-principles density functional theory (DFT-based calculations and ab initio molecular dynamics (AIMD) simulations can provide further insight into catalyst structure–property–performance relationships. More detailed information regarding the application of in situ and operando S/TEM techniques for catalysis research can also be found in the articles in References 20,21,22,23.
Bright-field scanning transmission electron microscopy images showing surface morphological changes of a Pt3Co nanoparticle catalyst during atmospheric oxidation at 350°C.18 Utilization of in situ gas cells permits atomic-scale observations of gas-phase chemical reactions at elevated temperature and at full atmospheric pressure. Reprinted with permission from Reference 18. © 2017 American Chemical Society.
Another research area that has benefited from the development and application of E-TEM and gas cell microscopy is the study of materials degradation processes under extreme environments. Oxidation and high-temperature corrosion are leading causes of premature failure in metals and alloys that are used in structural applications. To design corrosion resistant alloys, understand their behavior in aggressive environments, and to predict service lifetime, understanding corrosion mechanisms and identifying distinct microstructural features such as grain boundaries, carbides/precipitates, and secondary phases that lead to corrosion are critically important. Corrosion or oxidation initiation sites occur at the atomic to nanoscale; therefore, S/TEM characterization is a powerful technique that can pinpoint and correlate susceptible microstructures to the corrosion behavior. Identifying corrosion mechanisms and measuring kinetics has often been conducted through postmortem characterization techniques, wherein characterization is performed after the material has been subjected to corrosive environments. The article by Zhou et al. in this issue discusses how materials degradation mechanisms can be better understood through E-TEM and in situ gas cell approaches.24 For example, the oxidation behavior of pure copper metal was shown to occur through monolayer adatom growth mechanisms of Cu2O at terraces along (110) crystal orientation.25 β-NiAl oxidation kinetics was measured in situ as a function of temperature and near atmospheric pressure using a gas cell system where it was determined that Al2O3 oxide scale formation is rate-limited by Al3+ and Ni2+ cation diffusion.26 Figure 3 shows an example of in situ E-TEM experiments that were performed to fundamentally understand step-edge and subsurface oxidation mechanisms of NiAl in O2 and H2O environments, at atomic resolution.27 With more access to this type of instrumentation, more experiments are expected to be performed and breakthroughs in determining age-old corrosion mechanisms will be forthcoming.
In situ atomic-scale imaging reveals the difference in oxide nucleation and growth behavior along the (110) interface of Ni–Al during oxidation at 350°C in pure O2 (a, b) and within the presence of H2O vapor (c, d). Scale bars = 1 nm. Because the environmental-transmission electron microscope experiments reveal these fundamental mechanisms at atomic resolution, experimental results are better corroborated with density functional calculations and ab initio molecular dynamics simulations. Reprinted with permission from Reference 27. © 2020 AAAS.
Conclusions
In situ/operando gas-phase microscopy research has steadily advanced over decades of R&D to the point where now it is a mainstream materials characterization technique with commercially available E-TEMs and in situ gas-cell holders available. The clear advantage of this technique is the ability to conduct experiments where dynamic gas-phase chemical reactions can be imaged and analyzed at or near atomic resolution and under a range of environmental conditions. Thus far, the technique has provided new levels of detail regarding materials synthesis and materials behavior under extreme environments. In the near future, we fully expect that more detailed insight and innovative use of high-speed cameras/detectors for increased temporal resolution, application of 4D-STEM-based techniques (e.g., strain mapping, differential phase contrast imaging, and electron ptychography), automated experiments, and data analytic workflows based on artificial intelligence and machine learning will be applied simultaneously with in situ/operando gas-phase experiments.28
References
P.R. Swann, N.J. Tighe, Jernkontorets Annaler 155, 497 (1971)
E.D. Boyes, P.L. Gai, Ultramicroscopy 67, 219 (1997). https://doi.org/10.1016/S0304-3991(96)00099-X
J.F. Creemer, S. Helveg, P.J. Kooyman, A.M. Molenbroek, H.W. Zandbergen, P.M. Sarro, J. Microelectromech. Syst. 19(2), 254 (2010). https://doi.org/10.1109/jmems.2010.2041190
L.F. Allard, S.H. Overbury, W.C. Bigelow, M.B. Katz, D.P. Nackashi, J. Damiano, Microsc. Microanal. 18(4), 656 (2012). https://doi.org/10.1017/S1431927612001249
T. Yaguchi, M. Suzuki, A. Watabe, Y. Nagakubo, K. Ueda, T. Kamino, J. Electron Microsc. 60(3), 217 (2011). https://doi.org/10.1093/jmicro/dfr011
T.W. Hansen, J.B. Wagner, Controlled Atmosphere Transmission Electron Microscopy (Springer, Cham, 2016)
F.M. Ross, J. Tersoff, M.C. Reuter, Phys. Rev. Lett. 95, 146104 (2005). https://doi.org/10.1103/PhysRevLett.95.146104
R. Sharma, Z. Iqbal, Appl. Phys. Lett. 84, 990 (2004). https://doi.org/10.1063/1.1646465
H. Yoshida, S. Takeda, T. Uchiyama, H. Kohno, Y. Homma, Nano Lett. 8, 2082 (2008)
C.-Y. Wen, M.C. Reuter, J. Bruley, J. Tersoff, S. Kodambaka, E.A. Stach, F.M. Ross, Science 326(5957), 1247 (2009). https://doi.org/10.1126/science.1178606
F. Panciera, Z. Baraissov, G. Patriarche, V.G. Dubrovskii, F. Glas, L. Travers, U. Mirsaidov, J.-C. Harmand, Nano Lett. 20(3), 1669 (2020). https://doi.org/10.1021/acs.nanolett.9b04808
K.A. Dick, MRS Bull. 48(8) (2023)
H. Yoshida, Y. Kuwauchi, J.R. Jinschek, K. Sun, S. Tanaka, M. Kohyama, S. Shimada, M. Haruta, S. Takeda, Science 335, 317 (2012). https://doi.org/10.1126/science.1213194
W. Yuan, B. Zhu, K. Fang, X.-Y. Li, T.W. Hansen, Y. Ou, H. Yang, J.B. Wagner, Y. Gao, Y. Wang, Z. Zhang, Science 371(6528), 517 (2021). https://doi.org/10.1126/science.abe3558
J.F. Creemer, S. Helveg, G.H. Hoveling, S. Ullmann, A.M. Molenbroek, P.M. Sarro, H.W. Zandbergen, Ultramicroscopy 108(9), 993 (2008). https://doi.org/10.1016/j.ultramic.2008.04.014
S.B. Vendelbo, C.F. Elkjaer, H. Falsig, I. Puspitasari, P. Dona, L. Mele, B. Morana, B.J. Nelissen, R. van Rijn, J.F. Creemer, P.J. Kooyman, S. Helveg, Nat. Mater. 13, 884 (2014). https://doi.org/10.1038/nmat4033
T. Ghosh, J.M. Arce-Ramos, W.-Q. Li, H. Yan, S.W. Chee, A. Genest, U. Mirsaidov, Nat. Commun. 13, 6176 (2022). https://doi.org/10.1038/s41467-022-33304-x
S. Dai, Y. Hou, M. Onoue, S. Zhang, W. Gao, X. Yan, G.W. Graham, R. Wu, X. Pan, Nano Lett. 17(8), 4683 (2017). https://doi.org/10.1021/acs.nanolett.7b01325
M. Willinger, T.W. Hansen, MRS Bull. 48(8) (2023)
F.F. Tao, P.A. Crozier, Chem. Rev. 116, 3487 (2016). https://doi.org/10.1021/cr5002657
H.-Y. Chao, K. Venkatraman, S. Moniri, Y. Jiang, X. Tang, S. Dai, W. Gao, J. Miao, M. Chi, Chem. Rev. 123(13), 8347 (2023). https://doi.org/10.1021/acs.chemrev.2c00880
T.W. Hansen, J.B. Wagner, ACS Catal. 4, 1673 (2014). https://doi.org/10.1021/cs401148d
P.L. Gai, E.D. Boyes, S. Helveg, P.L. Hansen, S. Giorgio, C.R. Henry, MRS Bull. 32(12), 1044 (2007). https://doi.org/10.1557/mrs2007.214
G. Zhou, K.A. Unocic, C. Wang, Z. Shan, S.J. Haigh, J.C. Yang, MRS Bull. 48(8) (2023)
G. Zhou, L. Luo, L. Li, J. Ciston, E.A. Stach, J.C. Yang, Phys. Rev. Lett. 109, 235502 (2012). https://doi.org/10.1103/PhysRevLett.109.235502
K.A. Unocic, D. Shin, R.R. Unocic, L.F. Allard, Oxid. Met. 88, 495 (2017). https://doi.org/10.1007/s11085-016-9676-2
L. Luo, L. Li, D.K. Schreiber, Y. He, D.R. Baer, S.M. Bruemmer, C. Wang, Sci. Adv. 6(17), eaay8491 (2020). https://doi.org/10.1126/sciadv.aay8491
L.F. Allard, Y. Zhu, J.A. Dionne, S. Helveg, P.A. Crozier, J.R. Jinschek, MRS Bull. To be published.
Acknowledgments
This work was supported by the Center for Nanophase Materials Sciences (CNMS), which is a US Department of Energy, Office of Science User Facility at Oak Ridge National Laboratory (R.R.U.). E.A.S. acknowledges support by the National Science Foundation through the University of Pennsylvania Materials Research Science and Engineering Center (MRSEC) (DMR-1720530).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Unocic, R.R., Stach, E.A. Gas-phase electron microscopy for materials research. MRS Bulletin 48, 828–832 (2023). https://doi.org/10.1557/s43577-023-00588-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/s43577-023-00588-3