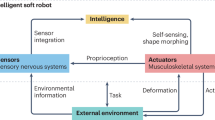
Abstract
Because plants are considered immobile, they remain underrepresented as concept generators for soft robots and soft machines. However, plants show a great variety of movements exclusively based on elastic deformation of regions within their moving organs. The absence of gliding parts, as found in the joints of vertebrates and insects, prevents stress concentration and attrition. Since plants have no central control unit (brain), stimulus-sensing, decision-making and reaction usually take place noncentrally in the hierarchically structured materials systems of the moving organs, in what can be regarded as an example of physical intelligence. These characteristics make plants interesting models for a new group of soft robots and soft machines that differ fundamentally from those inspired by animals. The potential of such plant-inspired soft robots and machines is shown in six examples and is illustrated by examples applied in architecture and medicine.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
Introduction
Movement and adaptive motion represent characteristic aspects of life and are found in representatives of all organismal kingdoms. A look at the involved processes in living beings and especially in plants and animals makes it obvious that such processes are characterized by the deep integration of the actuating (motor) parts in the moving body and a genuinely embedded elasticity of motion. Among many other aspects, this fundamentally distinguishes motion processes in living organisms from those of technical devices, which are characterized by a separation of the actuating/propelling parts from the moving body. In technical devices, typically rigid structures are moved with rotating wheels.
Motion is usually perceived by humans to be associated with animals, whereas plants are commonly considered as being largely immobile. This is because the visible changes in the position of moving animals normally take place within time scales from tenths of seconds to several seconds making them easily recognizable for human visual perception. From an evolutionary point of view, this recognition was probably important for the survival of early humans who were hunters/gatherers and sometimes the prey of other animals.
As a consequence of the internal structure of limbs in vertebrates and arthropods, motion causes (1) stress concentration in the localized gliding joints and (2) wear attributable to friction, making these joints prone to fatigue and failure. In all of these mentioned aspects, plants differ from the previously discussed animal groups, which represent the most important models for “conventional” (i.e., non-soft) bioinspired robots. Plant movements are based on the elastic deformation of larger regions of the moving organ itself, without any gliding parts being involved. Therefore, stress concentration and wear from friction are largely avoided or at least markedly reduced. On the one hand, visible changes in position during plant motion typically take place (very) slowly within time scales between several minutes (e.g., the opening and closing of pine cones) to hours (e.g., the circumnutation of searcher twigs and tendrils of lianas) to days or weeks (e.g., growth processes). On the other hand, some plant motions are extremely fast and happen within milliseconds (e.g., in motile traps of carnivorous plants) or even microseconds (e.g., spore discharge in ferns).1,2 Both time scales make the recognition of plant movements by our visual system difficult, if not impossible. This could be the main reason that plants are mostly considered as static and are still underrepresented as concept generators for soft robots and soft machines, despite their fascinating motion patterns and their low error-prone bending zones.3,4
To understand the potential of plants for soft robotics, we need to have a closer look at the three basic types of plant movements: tropisms (e.g., phototropism, geotropism, thigmotropism, chemotropism); nastic movements (e.g., thigmonastism, seismonastism, hydronastism, photonastism); and autonomous movements. Tropistic movements are mediated mostly via growth processes and are relatively slow. They represent vectorial movements, in which the direction of reaction is determined by the direction of the stimulus. Nastic plant movements can be much faster (especially if stored elastic energy is released) and are actuated typically by turgor changes or swelling/shrinking processes attributable to humidity changes (e.g., pine cone opening and closing, snap-trapping in the Venus flytrap). The direction of reaction is independent of the direction of the stimulus, but is predetermined by the structure and form of the reacting organ. Autonomous movements, which are induced by internal stimuli, are typically mediated via growth processes and/or turgor changes. They are often of medium speed (e.g., the circumnutations of the searcher stems and tendrils of lianas).1,2,5 Currently, nastic plant movements are by far the most dominant concept generators for plant-inspired soft machines or soft robots. In contrast, tropistic movements are rarely used, but are of particular interest for adaptive motion.6,7,8
In order to achieve the successful transfer of plant motion principles (including their material-based sensing and actuation mechanisms) to biomimetic soft robots or soft machines, detailed analyses of the form-structure–function relationships of the involved plant tissues and organs and their inherent materials systems are indispensable. Materials systems in plants are characterized by their scale-overarching mechanical properties, their hierarchical structuring comprising up to 10 orders of magnitude.9 Interesting plant-inspired motile devices include materials systems with high load-bearing capacity at little mass and various self-induced properties (e.g., self-repair or self-cleaning ideally with material-based sensing).3
From the multitude of plant structures that can serve as models for motile bioinspired structures, we focus on six examples in this article. Pine cone scales and silver thistle bracts, which both show nastic hygroscopically actuated multiphase motion, serve as models for self-adaptive protection flaps in building hulls and in a self-locking soft machine. Nastic turgor-driven motion with or without speed-boosting mechanisms have been the inspiration for pneumatically actuated cellular actuators and a novel type of artificial Venus flytrap (the latter having speed-boosting mechanisms). Autonomous movements in liana searcher stems and tendrils serve as concept generators for bioinspired robot arms, artificial tendrils and a novel self-adaptive orthotic device. In these cases, actuation modes differing from those in the biological models (e.g., growth, turgor changes, lignification) have been chosen during the biomimetic transfer process.
Hygroscopic plant motions: Movement without consumption of metabolic energy
Plants are able to react to environmental stimuli, such as light or touch, with a variety of movement responses. A famous example is the Venus flytrap, which, upon being touched by an insect, rapidly shuts its snap-trap so that the animal is caught,10 only reopening the trap after digestion or an unsuccessful attempt.11 This iconic snap-trapping motion has served as an inspiration for various types of artificial Venus flytraps (see “Novel artificial Venus flytrap systems: Going beyond biology” section). Many of these responses are costly for the plants in terms of the consumption of metabolic energy. However, plants have also evolved a strategy enabling them to move in response to humidity changes as the environmental trigger, but without investing metabolic energy. This “cost-free” adaptivity is rendered possible by the development of hygroscopic tissues consisting of dead cells, which can passively take up water from the environment (entailing swelling) and can later release it via evaporation (entailing shrinkage). The fine architecture of the individual hygroscopic cells and cell walls dictate the water-driven deformation at the microscale, whereas the form and interplay of tissues with their different swelling/shrinking properties are responsible for the meso- and macroscale movement responses of the respective plant structure.12,13 Plants have evolved hygroscopically driven motions in a multitude of organs, which are often involved in the release and dispersal of seeds and spores, such as capsules, cones and awns (e.g., References 14 and 15). All hygroscopic plant motions represent nastic plant movements with a predetermined motion pattern and sequence.
Such hygromorphs are “classically” described as bilayer structures in the literature, each incorporating a hygroscopically active layer and a passive resistance layer,16,17 which, when fixed together, produce bending upon changes in humidity. However, a recent study has shown that a classical textbook example of hygroscopic bilayer actuation, namely the seed scale of pine cones, is indeed a much more complex composite structure consisting of numerous functionally important layers.15 Each pine cone features along its axis numerous helically arranged seed scales that all bend downward when it is dry (entailing the opening of the cone and seed release) and bend upward under wet conditions (entailing cone closure with safely enclosed seeds) (Figure 1a). The closure of the cone is initiated by water entering the individual seed scale primarily through its adaxial epidermal tissue. The water is then taken up by the so-called brown tissue and sclerenchyma strands and passively transferred to the rest of the scale by diffusion and the locally increased pressure of the swollen tissues. The sclerenchymatous strands additionally form the resistance layer and soften upon swelling, leading to a mechanical “unlocking” of the scale and enhancement of its compliance. The water finally reaches the sclereid layer, which is very prominent at the basal part of the scale (termed the bending zone). The sclereid layer swells predominantly in a longitudinal direction, thereby driving the bending of the scale. In summary, the four tissue types involved in the motion of the seed scale of the pine cone have different functions and act as motors (sclereids), water barriers and transducers (epidermises, brown tissue, and sclerenchyma strands) and a resistance layer with hydraulically switchable mechanical properties (sclerenchyma strands) (Figure 1b). Recent studies show that the sclerenchyma strands themselves may additionally act as actuators increasing and speeding up the scale movement.18
Pine cone actuation, functional morphology, and biomimetic self-sufficient compliant systems. (a) A pine cone shows sequential seed scale movements during the drying-induced opening sequence. The time scale is indicated. Figure adapted from Reference 19. (b) Schematic top view (left) and longitudinal section (right) of a pine cone seed scale. The various, functionally important, tissue types (epidermises, sclereids, sclerenchyma, brown tissue) are indicated with a color code. The bending zone at which the actuating sclereid tissue is most prominent is highlighted by the gray box. Figure adapted from Reference 15. (c) Multiphase motion of a 4D printed scale structure consisting of copolymer strands with embedded cellulose fibrils as the actuating layer (analogous to the actuating sclereid layer in the natural seed scale) and ABS polymer strands as the resistance layer (analogous to the sclerenchymatous strands). The structure performs reversible sequential motion steps during drying (i.e., first, a change of transverse curvature [flattening of the artificial scale] and, afterward, a change of longitudinal curvature). Image adapted from Reference 21. (d) Vision of a modular smart-building skin consisting of shingle-like structures exhibiting tropistic behavior for sun-tracking behavior (to give optimal shading) and nastic behavior for fast hull closure upon an increase of environmental humidity. Figure adapted from Reference 24.
Alterations in tissue dimensions and in overall scale size and morphology result in various movement behaviors, which is most presumably one of the reasons for the orchestrated pine cone opening and closing processes with sequential scale bending motions. Similarly, orchestrated motions can be found, for example, in the inflorescence heads of the silver thistle (Carlina acaulis ssp. simplex) whose surrounding involucral bracts bend sequentially toward the head upon an increase of humidity, thereby protecting the central flower head.19 The strict morphological predetermination of such so-called nastic hygroscopic plant motions in terms of their movement patterns and time scales, their complete detachment from any influencing metabolic processes and their functional resilience20,21 make them excellent concept generators for biomimetic approaches. Numerous studies, especially in the field of biomimetic architecture, have shown that self-sufficient compliant systems (e.g., for smart building hulls) can be achieved using natural and/or (bioinspired) technical bilayer materials systems.19,22,23 However, problems inherent to many layered hygro-actuators are (1) the occurring moisture gradients; (2) the delamination of the materials systems caused by repetitive swelling and shrinking cycles (i.e., the separation of the functional layers during deformation); (3) their slow response times attributable to the passive process of water displacement through the motile structures, especially when they are of architecturally relevant dimensions; and (4) limitations attributable to increases in the weight of the actuated structure. These topics and issues have been discussed and possible future research approaches for their solution are proposed in Poppinga et al.24
Novel 4D printed scale structures capable of multiphase movement sequences20 (Figure 1c) and compliant systems showing tropistic motion responses (i.e., light tracking) with their motion depending on the direction of the triggering stimulus have recently been developed.25 Further inspiration taken from pine cone architecture could make it possible in future approaches to develop self-sufficient compliant systems consisting of numerous programmable functional layers with short response times, exhibiting multi-responsiveness (i.e., sensitivity to various stimuli), precisely fine-tuned stimulus thresholds and high functional resilience and combining nastic and tropistic movement behavior. In the sketch presented in Figure 1d, we envisage a smart biomimetic building hull that regulates the climate of the building through autonomous shading and “breathing” responses during various weather regimes by such a combination of nastic (humidity-dependent) and tropistic (light-direction-dependent) movements. Furthermore, the mechanical coupling of actuator domains (i.e., arrays of several actuating units) represents a promising aspect for future studies aimed at gaining a better understanding of kinematic amplification principles.
Liana stems and tendrils as inspiration for actuators and self-lashing systems
Lianas with their specific non self-supporting growth habit, which relies on various types of attachment to their host structure,26 provide many ideas for technical transfer. These include orthotic devices inspired by the twining attachment of liana stems (see “Technical transfer and computational design of liana-inspired medical orthoses and self-locking mechanism inspired by silver thistle bracts” section) and robot arms inspired by the intertwining of liana searcher stems. These braided searchers enable not only a wider reach with reduced material investment, but also a discrete change in flexural stiffness along the braid. Flexural stiffness increases in a stepwise fashion, with each additional stem increasing stiffness by more than two orders of magnitude from the apical single leading searcher stem to the base of the braid formed by four intertwined searcher stems.27
In addition to twining around a support or using adhesive roots,28,29,30 many plants attach to a support via coiling tendrils (contact coiling) or attachment pads31,32 that “grasp” or affix themselves to the support, respectively. Once they are attached, these tendrils can also coil along their own axis (free coiling) (Figure 2a) (e.g., Reference 33). Thereby, they shorten, pull the plant closer to its support and form a spring-like and energy dampening structure (e.g., References 32 and 34). Tendrils have long attracted the interest of scientists,35,36,37 with biologists continuing to unravel the functional principles behind their movements. Recently, tendrils have also attracted the attention of soft roboticists who have mimicked contact coiling for grasping38,39,40 and axial shortening by free coiling for actuators.38,39,41,42,43,44
Tendrils of the passion flower P. caerulea in the premature state (i.e., uncoiled [left] and in the mature state showing contact and free coiling [right]). The insets show cross sections of the tendrils at three different developmental stages during which the central tissue lignifies (cross sections: red = lignin-specific phloroglucinol-hydrochloric acid straining) (a). Passiflora discophora stem attached via multiple tendrils showing adhesive pads (b) that act together when the stem is placed under tension (c) and thus allow the plant to sustain higher forces before failing/detaching than would be the case for individual tendrils (d, e). Thick black arrows indicate direction of force application. Figure (d) adapted from Reference 31.
Plant tendrils coil because of a difference in length between their opposing sides, an effect produced by osmotic effects (turgor change, [i.e., water displacement]), differential growth33,35,36,45 or contractile fibers.46,47 During free coiling, the intrinsic curvature of the tendrils created by the length difference causes the formation of a particular spring-like shape: The tendril falls into a minimum of two helices of opposite handedness.48,49 We have observed that, during the free coiling of the tendrils of the blue passion flower (Passiflora caerulea), their central tissue begins to lignify (and thus to stiffen) unilaterally (i.e., asymmetrically) (Figure 2a), indicating that differential tissue maturation also contributes to coiling. In older tendrils, the central cylinder is fully lignified, fixing the tendril in the coiled position. Finally, the tendril dies (senescence), but remains mechanically functional,31 without further costs of metabolic energy.
As in the biological model, coiling in many technical and biomimetic implementations is based on a strain mismatch between two materials. To this end, Cheng et al.,42 Gerbode et al.,47 and Meder et al.40 report bonding a prestrained silicone rubber ribbon to a relaxed ribbon, with the formation of a helix upon release of the prestrain. The tendril developed by Meder et al.40 has a polycaprolactone core surrounded by a resistive heating element embedded in a relaxed silicon elastomer layer that controls the spontaneous coiling of the out-of-equilibrium bilayer by a solid-to-liquid phase transition upon heating (Figure 3a). Cheng et al.42 have incorporated a conductive polyester yarn into a non-prestretched layer that contracts like a spring when a current is applied (Figure 3b). Instead of using (prestretched) silicone rubber ribbons, Kanik et al.44 make use of the different coefficients of thermal expansion of a high-density polyethylene (PE) layer bonded to a cyclic olefin copolymer elastomer (COCe) layer in a thermal drawing process. Inducing plastic deformation in the PE layer in a subsequent cold drawing process results in a coiled spring that can then be actuated by heating. The robotic tendril presented by Hu and Alici43 is also based on a strain mismatch between two layers comprising, in this case, a pneumatically actuated extensible layer and an inextensible layer.
Various types of artificial tendrils. Tendril consisting of a silicon elastomer bilayer with an embedded polycaprolactone (PCL) core surrounded by a resistive heating element that controls spontaneous coiling of the out-of-equilibrium bilayer by a solid-to-liquid phase transition (a) (adapted from Reference 40). Tendril consisting of silicon elastomer bilayer with an embedded polyester yarn that contracts when a current is applied (b) (adapted with permission from Reference 42. © 2018 American Chemical Society). Setup for producing nanoscale helical fibers via off-centered core–shell co-electrospinning (c) (adapted with permission from Reference 50. © 2015 American Chemical Society). Tendril-inspired gripper actuated using an osmotic strategy based on the electrosorption of ions (d) (adapted with permission from Reference 39).
Material programming and 4D printing of bioinspired self-shaping materials systems based on the fabrication parameters of FFF-based 3D printing. (a) Processing parameters include the nozzle size N, layer height H, and toolpath spacing of the active A and restrictive R filaments and the resulting extrusion paths P. Bending can be tuned via the mesostructure design: for example, (a) the amount of curvature can be set and (b) various curvatures can even be combined together in different configurations (c). Figure adapted with permission from Reference 56.
Although not being a bilayer in the true sense of the word, a structure that is similar in terms of its internal design has been produced by Wu et al.50 using an off-centered core–shell co-electrospinning system. The nanoscale helical fibers consist of a poly(m-phenylene isophthalamide) (Nomex) core in a polyurethane shell (Figure 3c). Even though the primary application of these fibers is described as being in nonwoven material, one can well imagine their use in the field of soft robotics. The production of helical structures by electrospinning is well reviewed by Silva et al.51 A different approach, which is not based on bilayers, relies on nickel-titanium (NiTi) shape-memory alloys for producing technical tendrils.38,41 Yet another principle uses an osmotic strategy based on the electrosorption of ions to actuate (“inflate”) a preformed tendril-inspired gripper39 (Figure 3d).
This brief and certainly not exhaustive overview demonstrates the great interest in artificial tendrils. Surprisingly little attention, however, has been paid to the way that multiple tendrils interact. Figure 2b shows that the alternating individual tendrils each pull the stem of the passion flower P. discophora closer to the points of fixation, resulting in the conspicuous zig-zag shape that highlights how tightly the stem is lashed to the substrate. When pulling on a stem, we find that even tendrils more than 1.5 m away from the point of force application are strained and, hence, that the load and energy dissipation are distributed between multiple tendrils that act together. Indeed, our quantitative tensile tests on stem segments bearing multiple tendrils (Figure 2c) clearly show that their failure force is well above that of individual tendrils (Figure 2d, e).31 The tendrils are therefore not loaded sequentially and fail one after the other; on the contrary, they act together in a parallel coupling. The arrangement of multiple tendrils not only increases the force necessary for stem detachment, but additionally represents a “fail-safe-system” with high energy being needed before detachment. We assume that the coiled appearance of these tendrils is not only advantageous for the dissipation of energy, but also makes the interaction and simultaneous loading of several tendrils possible. The use of completely straight tendrils would require their lengths to be well harmonized, a difficult feat to achieve in both plant and technical implementations. A novel type of self-coiling tendril is currently being developed in an interdisciplinary project with the aim of testing our predictions concerning the interaction of natural tendrils with a system of artificial tendrils.52
Much can still be gleaned from the biological models, especially with regard to the interaction of several (actuated) anchoring systems. For instance, the maintenance of reversible actuability is often not necessary and a two-stage process can be programmed in which the anchor obtains its final mechanical properties only after the termination of the movement. Further, we can reasonably rely on several (cheap) anchor points, rather than trying to perfect individual anchors. This is especially important if the substrate is unpredictable, as in safety nets, for example.
Technical transfer and computational design of liana-inspired medical orthoses and self-locking mechanism inspired by silver thistle bracts
Advances in computational design and digital fabrication have enabled the technical transfer of various plant functions to our engineered systems. In particular, additive manufacturing has been proven to be well suited for preprogramming spatial and temporal movements that are triggered in response to environmental stimuli (a process termed 4D printing53) by emulating the features of motile plant structures at increasingly high resolutions and accuracy.
The fused filament fabrication (FFF) method is ideal for producing bioinspired self-shaping materials systems. Not only are the printing technology and filament materials low cost and widely accessible, but the extrusion process of FFF inherently creates mesoscale directional features through the paths of material deposition. By controlling the extrusion of natural fiber composites in the FFF process, it is possible to encode an anisotropic and hygroscopic structure analogous to the cellulose microfibrils in plants, allowing their passive movements to be mimicked in 4D printed materials systems.54
Through a material-programming and 4D-printing framework developed for choreographing the sequence and quality of filament deposition, a combination and range of materials can be precisely positioned at the mesoscale, resulting in a multimaterial and multilayered mesostructure.55 Variations in the trajectory pattern, printing layer height, spacing between extrusion paths and flow of extrusion have a compounded impact on the resulting directional stiffness and elasticity of the materials system and on its bending direction, orientation, and magnitude (Figure 4a–c). By utilizing the intuitive geometric descriptors from existing CAD workflows, individual functional regions within a networked assembly can be assigned with specific materials properties to be directly translated into the fabrication data necessary for producing the desired self-shaping behavior. Thus, this integrated computational design approach allows the construction of intricate mesostructures (0.5 mm resolution) within macroscale parts (as large as 300 mm), while simplifying the complexity of designing highly differentiated materials systems.
Fundamentally, the ability to tailor the mesostructure expands the possibilities for the further transfer of functional principles from biological structures to 4D printed materials systems. The bilayer structure of the pine cone scale has been transferred to composite systems at an abstracted level enabling bending in a single curvature and orientation.18 However, 4D printing allows the programming of consecutive motions such as the transversal and subsequent longitudinal bending transformation observed in pine cone scales.21
Besides the pine cone are a myriad of plant role models with functionalities yet to be transferred to technical systems. The flower head (inflorescence) of the silver thistle (Carlina acaulis ssp. simplex), for example, is surrounded by rows of bracts that expose the inner inflorescence for pollination during dry and favorable conditions. In wet conditions, the rows of bracts bend inward in a sequential manner, fully shielding the inflorescence to protect it from rain (Figure 5a). This sequential movement has been studied in 4D printed materials systems by tuning the mesostructure porosity and thickness and by adding a blocking layer with tunable moisture permeability.19 Four-dimensional printing has thus been shown to modulate successfully the speed of actuation separately from the desired curvature (Figure 5b), thereby allowing similar curvatures to be reached in dramatically different time scales under the same actuation conditions, as demonstrated in a self-locking soft machine in which the timing becomes critical for two parts to intersect without colliding (Figure 5c).
(a) Sequential shape-change in the silver thistle (C. acaulis ssp. simplex). (b) A 4D printed aperture with six flaps taking turns to bend until completely opened. (c) A 4D printed soft machine is shown inserting its slot into the locking flaps. Figure adapted with permission from Reference 19.
Plants have evolved a multitude of clever strategies for their survival, some of which have enormous potential for abstraction and transfer to the technical domain in order to provide a solution to a problem. The air potato (Dioscorea bulbifera) is a climbing plant that has been observed to twine around smooth supports while squeezing them with considerable force to prevent slipping as it ascends to great heights. The mechanism of force generation has been found to originate from the delayed expansion of stipules at the leaf base; these stipules stiffen the helical stem through tensioning and markedly increase the contact forces applied to the support around which the stem is twined.28 As the quality of squeezing is strong and yet smooth and continuous, this two-phase tensioning mechanism presents a promising opportunity for improving the comfort and fit of assistive wearable devices that need to adapt slowly over time. As such, 4D printing has been used to construct an integrated material system comprising two sub-mechanisms (Figure 6a) reproducing the twining and tensioning behavior exhibited by the role model.56 D. bulbifera naturally exhibits stipule growth at discrete points, spaced approximately three gyres apart on the stem. However, a 4D printed bioinspired orthotic splint has been customized with an increased number of tensioning mechanisms programmed with further actuation delay (Figure 6b) resulting in higher squeezing forces than found in nature and demonstrating the functional possibilities of computational design.
(a) The biomimetic process starting from the investigation and understanding of D. bulbifera to the production of a bioinspired 4D printed material system and wearable application. (b) A close-up of the many tensioning mechanisms of the 4D printed orthotic splint prototype; the mechanisms are populated at a higher density than that occurring naturally in the plant role model, illustrating the advantages of the computational design framework. Figure adapted with permission from Reference 56.
Potential applications of bioinspired 4D-printing extend beyond adaptive orthoses to self-shaping architectural elements. Indeed, the reduction of operational energy related to regulating the interior climate of buildings represents a major ecological challenge.57 In light of the increasing demand for energy efficiency, current work is underway on the development of the next generation of bioinspired adaptive shading elements for weather-responsive building envelopes.58 Four-dimensional printed self-shaping materials systems that operate autonomously in response to natural shifts in environmental conditions, requiring neither electromechanical devices nor operational energy, represent a step toward a more resilient and sustainable future.
A cellular actuator inspired by bulliform cells of grass leaves
From the variety of plant motions movable but hinge-less structures are of particular interest, because they can serve as suitable models for technical developments that guarantee a long service life without costly maintenance or replacement of broken hinges. A prime biological model for durable technical applications is the hinge-less motion of grass leaves triggered by the turgor changes of their bulliform cells.59,60 Within the framework of a biomimetic technology pull process, Mader et al.61 have abstracted this biological principle and applied it to a pneumatically driven biomimetic cellular actuator for facade-shading systems at the architectural scale.
In the grass families Poaceae, Juncaceae, and Cyperaceae, fan-shaped groups of huge epidermal cells (so-called bulliform cells or motor cells) occur on the adaxial (= upper) side of the leaves (Figure 7a,b). If the groups of bulliform cells are concentrated either directly on the midrib of the grass leaf or in two strands left and right close to the midrib, the leaf halves exhibit a cross-sectional V-profile. The leaf halves are closed under drought stress and can open again under sufficient water supply. When the bulliform cells become turgescent and increase their volume by swelling, the leaf halves open around the midrib.60 Damage experiments of the bulliform cells support the hypothesis that prestresses in the closed leaf, caused by the other leaf tissues, have to be actively counteracted by a high turgor pressure in the bulliform cells in order to open the leaf.61 The leaf halves close again when the turgor is low and the volume of the bulliform cells shrinks.60 Experiments on maize leaves indicate that the cuticles of bulliform cells are more permeable to water than the cuticles of the other epidermal cells. Thus, during leaf dehydration, bulliform cells shrink more than the epidermal cells.62 The pivotal region of the motion is located in the midrib area. Vascular bundles together with sclerenchyma struts between the upper and lower leaf epidermis serve as spacers giving the leaf lamina its integrity (Figure 7a-b).
(a) Unstained cross section of an entire leaf of S. nitida. The purple rectangle indicates the image detail shown in (b). (b) Microscopic image stained with Acridine Orange that highlights lignified tissues in yellow. The contours of the leaf and the bulliform cells are outlined in black. Note: ab-ep, abaxial epidermis (= lower epidermis); ad-ep, adaxial epidermis (= upper epidermis); bc, bulliform cells; ch, chlorenchyma; mr, midrib; sc, sclerenchyma; sc-mr, midrib sclerenchyma; vb, vascular bundle; vb-mr, midrib vascular bundle (= midvein). (c) Finite element model of one leaf half opening and closing dependent on the turgor pressure (P) in the fan-shaped bulliform cell group.
This turgor-dependent motion and change in the geometry of grass leaves is currently interpreted as being a reversible response to water limitation and heat stress. Under drought stress, which causes low turgor, the folding reduces the exposed leaf surface and thus transpiration and protects the leaves from dehydration and overheating. Because the stomata in grass leaves are located on both the upper and lower side of the leaves, folding can significantly reduce free transpiration from the stomata on the adaxial side. When the water supply is sufficient (i.e., at a high turgor), the leaf is unfolded and flattened (Figure 7a-b). This increases photosynthesis because of the higher sun exposure of the leaf surface. Moreover, folding of the leaf also has a mechanical side effect as to its flexural rigidity under gravitational loading. Compared to an entirely flat and fully turgescent leaf (width a ~ 6.0 mm, average height b ~ 0.335 mm) a completely folded dehydrated leaf (height a/2 ~ 3.0 mm, width 2b ~ 0.67 mm) has an approximately 80 times higher axial second moment of area in direction to gravitational loading. Additionally, it can be assumed that the elastic modulus of the entire leaf structure does not change markedly under drought stress as turgor-independent sclerenchyma plays the major stiffening role in terms of struts between the upper and lower epidermis. Thus, we hypothesize that the increase of the axial second moment caused by leaf folding overcompensates for the (slight) decrease in elastic modulus and the folded leaf is markedly stiffer in bending under gravitational loading than the unfolded, but turgescent leaf.
The turgor-triggered motion in the grass leaves represents (like the hygroscopic movements) a nastic movement with a predetermined motion sequence.63,64 In contrast to the hygroscopic movements of the “dead” plant tissues described in “Hygroscopic plant motions: Movement without consumption of metabolic energy” section, metabolic energy is needed for the turgor changes initiating the motion in the grass leaves.
Mader et al.61 performed a detailed form–structure–function analysis of the opening and closing motion of the leaves of Sesleria nitida, which belongs to the grass family (Poaceae). The motion driven by groups of bulliform cells arranged to the left and right of the midrib was the basis for the transference of this action into a technical motile structure. Mader et al.61 incorporated all essential insights, such as geometry, shape and arrangements of the bulliform cells and the pressure-dependency of the motion, into a finite element analysis as a prerequisite for the development of a biomimetic pneumatic cellular actuator (Figure 7c). The first prototype of the cellular actuator consisted of a row of single cells with compliant hinges positioned on a plate. When the pneumatic pressure applied to each individual cell was increased, the cells became wider at the upper side and the entire structure bent upward (Figure 8).
(a) Finite element model of a single technical cell. An increase in air pressure (P) inside the cell causes the vertical cell walls to tilt outward. (b) Finite element model of a row of cells under increased air pressure resulting in a bending motion. (c) Physical prototype of the biomimetic cellular actuator. The structure, consisting of a row of pneumatic cells attached to a plate, lifts an external mass of 0.5 kg (with reuse permission from Reference 61).
Because the size of the technical cells can span from centimeters to meters, the pneumatic cellular actuator has the potential for being applied on an architectural scale. To illustrate the feasibility of the concept as a bending actuator, the design and dimensions of the technical cells were chosen according to the prototype size of the bioinspired facade-shading system Flectofold.65,66 It has been successfully integrated into the midrib of the Flectofold, where the bending of its midrib controls the hoisting of its wings and, thus, the amount of shading.
In the framework of biomimetics, the functional principle of kinetic amplification has been transferred to a technical application without the copying of all details of the biological model. Therefore, similarities and dissimilarities can be found. The similarities between the plant model and the cellular actuator are the cellular structure, the fluid-mediated volume change of cells, and the reversible movement of the entire component. The dissimilarities are found with regard to the movement (folding of leaf halves versus bending of the cellular actuator), the fluid (hydraulically driven plant movement versus pneumatically driven deformation of single actuator cells), and the power (turgor pressure in the bulliform cells versus pressurized air in the technical cells).61
Novel artificial Venus flytrap systems: Going beyond biology
Over the last two decades, the mode of function of snap-trapping in Dionaea muscipula (Venus flytrap) has become much better understood and artificial Venus flytraps (AVFs) have increasingly moved into the focus of the R&D of bioinspired and biomimetic robots.67 If the trigger hairs on the trap lobes of D. muscipula are mechanically stimulated twice within a certain time frame (20–30 s at room temperature),68 the trap reacts with a snap-buckling and fast closure movement of its bistable lobes (within 500 ms)11,67,69,70,71,72,73,74 (Figure 9a). The trap’s fast movements, decentralized decision-making, mechanical memory, and energy-harvesting capabilities are of particular interest for use in autonomous robots, as these must also perform (fast) actions on an energy-efficient basis and gather energy from the environment.
(a) The biological role model D. muscipula (Venus flytrap) is shown in an open and closed state; the trap consists of two trap lobes connected by a midrib. If prey deflects the trigger hairs twice within a certain time, the trap lobes close in a fast snap-buckling trap motion. Figure a is adapted from Lin et al.75 under the terms of the Creative Commons Attribution License (CC BY). (b) Schematic overview of the actuation principles of the artificial Venus Flyflap.73 Contact-less magnetic actuation is achieved through a rotating magnetic field driving a magnet attached to one lobe ear in an up and down motion closing the artificial Venus flyflap (AVF) lobes periodically. In response to a rise in ambient temperature above 65°C, a spring made of a shape-memory alloy contracts and pulls the lobe ear down closing the trap lobes. A pneumatic drive is used to open and close the AVF repeatedly for motion analysis. A combination of humidity (>65% RH) and temperature (>65°C) unlocks the system for motion, as the bilayer of the shape-memory polymer and hydrogel becomes flexible and the hydrogel expands. In the dry and cold states, the system can be locked in an open state. (c) Pneumatic actuation has been used to characterize the five phasic opening and closing motions of the Venus Flyflap. (d) The opening motion is the fastest (<70 ms) of the five phases. Figure c and d adapted from Tauber et al.73 under the terms of the Creative Commons Attribution License (CC BY).73 (e) Side view of the unit cell-based AVF held at the central actuation unit. The system closes within 250 ms in response to a horizontal force being applied to the central unit. It inverses its lobe curvature, such as the Venus flytrap, passing a 0° curvature line (dashed line). Trap lobe motion paths are tracked and highlighted. (f) Force–displacement diagram during closure of the unit cell-based AVF. During curvature inversion, the force drops from 0.8 to 1.5 N down to 0.2 to 0.3 N with a force minimum after passing the 0° curvature line at 7 mm displacement. Different colors represent different demonstrators tested.
Esser et al.67 provided a detailed overview of AVF systems up until mid-2020. Since then, a few novel systems have been published highlighting new uses and production techniques for AVFs, such as snap-buckling joints for grippers,75 small-scale liquid–crystal elastomer grippers with magnetically printed liquid metal circuits for heating,76 and an AVF utilizing 3D printing to program multistability into structures morphing in response to changes in temperature.77
Such products were the inspiration for our novel AVF systems with which we were able to pass (partly) a Venus-flytrap functional Turing test when Venus-flytrap-like motion and energy requirements were considered.73,78 We benchmarked our systems to their biological role model and characterized them in terms of motion kinematics (motion path, closing time and speed, acceleration, etc.) and energy requirements.
Our first environmentally triggerable AVF system, the Venus Flyflap, combines, in a novel way, the motion principles of two carnivorous plants into one system. The AVF represents an example of a bioinspired development that “goes beyond biology.” The system closes like Aldrovanda vesiculosa (waterwheel plant) via the kinematic coupling of the trap lobes to the bending backbone,74 which represents the actuation system, causing a motion amplification, and snaps open in an “inverse” snap-buckling such as that of the Venus flytrap.73 The AVF is driven by four state-of-the-art actuation systems (Figure 9b): (1) pneumatic actuation closing and opening the trap lobes by pushing and bending the AVF midrib (Figure 9c); (2) thermal actuation via shape-memory springs closing in reaction to changes in ambient temperatures above 65°C; (3) a contactless magnetic drive via a permanent magnet attached to the closing structure moving up and down in response to a rotating magnetic field; (4) the locking of the system in an open state and its unlocking via a stimulus combination of humidity (>65% RH) and a rise in temperature (>65°C) utilizing a bilayer of shape-memory polymer and hydrogel.73
The pneumatically actuated AVF mimics and combines the motion principles of D. muscipula and A. vesiculosa and performs similarly to the biological models with regard to closing time and speed within its five phasic motions (Figure 9c). The system closes within 300 ms and snaps open within 60 ms (Figure 9d). The kinetic energy requirement for the motion (20 to 80 mJ) and the energy consumption of the actuation system (1 J for pneumatics)73 is less than the energy requirements of the biological models (9.66 J).70,79 The presented AVF system is the first developmental step toward a fully autonomous multi-responsive AVF system.
Our second system is the first bioinspired mechanical unit cell-based fully 3D printed AVF78 and originates from novel bending unit cells further developing the KinetiX system published by Ou et al.80 This mechanical metamaterial consists of rotating polygon type auxetic structures, enabling planar and spatial shape changes, such as uniform scaling, shearing, bending, and rotating, through a variation of position and the angle of hinges and plates. The system utilizes unit cells with oppositely titled walls to generate doubly curved surfaces mimicking the curvature and appearance of a Venus flytrap lobe. Two of these surfaces are connected by a central actuation unit cell mimicking the Aldrovanda midrib and form the AVF. The system is printed fully assembled and ready to use from the printer bed. By compressing the central unit in 2D by kinematic coupling, a 3D curvature inversion and closure of the trap lobes occurs within 250 ms (depending on the applied force) (Figure 9e).78 For curvature inversion, 0.8 to 1.5 N is needed with a force minimum after passing the 0° curvature line (Figure 9f). This novel unit cell AVF can be further miniaturized and will form the basis for an autonomous gripper and support structures for soft machines performing complex motion sequences in the future.
These systems can be employed in plant-inspired robotics as attachment, manipulation, or energy-harvesting structures equipped with photovoltaic elements within harsh environments.67 For such systems to cope with harsh environmental conditions, the research focus must lie in the development of a low complexity system with low wear and low to no maintenance. The novel AVF system presented here represents the first step toward fully autonomous structures for grippers and energy harvesters, as they already meet the requirements of low energy, low complexity, and autonomy. In addition, the two presented AVF systems represent the first systems capable of passing the Venus flytrap functional Turing test in motion kinematics and energy requirements.
Discussion and conclusion
Animal-inspired robots have a long history starting in the 1990s, with insect-inspired six-legged robots becoming increasingly “animal-like” in their motion sequences since the 2000s.81,82 Nevertheless, because of their modes of actuation and especially the use of mostly “hard and simple” solid materials, these artefacts differ markedly from their biological models, particularly with regard to details in their motion sequences. The development of soft robots inspired by, for example, octopus arms or plant roots started mainly in the 2010s and was a paradigm shift toward more life-like appearances and motions based on elastic deformation. “Soft” viscoelastic polymer-based materials and materials systems were mainly used for these robots.3,6,8,39,83,84,85,86 However, the types of actuation, which typically depend on external energy sources and wiring, showed only rudimentary autonomous material-immanent actuation and sensing, which is otherwise characteristic for the living concept generators.
Nevertheless, the functions of the biological models and, to an increasing amount, the underlying hierarchical structuring of the used materials systems were successfully mimicked, creating even more life-like types of robots. In the last five years, additional technically interesting functions of biological models (e.g., self-repair, self-adaptability, better sensor-actuator coupling, and even energy autonomy) have been incorporated in bioinspired soft robots and soft machines.3,6,8,83,84,86,87,88 For this next generation of soft robots, plant models have proven to be very helpful, especially because of the multifunctional materials systems of their motile structures. Such materials systems show a high level of structural and functional integration combining sensor, actuator, movable element and support structure and often displaying extraordinarily high functional resilience and robustness. The last-mentioned has been established for fossil char-coalified conifer cones, which hygroscopically open and close even after ~15 million years.20 Hygroscopic structures are prime models for energy autonomy as they harvest energy directly from the environment; in other words, they do not require metabolic energy (as in plants) or any wiring for external energy supply (as in soft machines). The (main) reasons for the evolution of these complex, widely autonomous, and sometimes energy-independent materials systems in plants are assumed to be their modular structure and their lack of a central control unit (i.e., a brain). These features necessitate that sensing and (re)acting take place in a decentralized manner across the various modular plant organs or structures, making plants highly appropriate concept generators for novel smart materials systems. Such types of hierarchically organized and highly functionally integrated plant-inspired materials systems can be seen as a prerequisite for the next generation of increasingly autonomous, self-sufficient, soft robots and soft machines.4 State-of-the-art production methods such as 3D/4D-additive (micro-) manufacturing, 3D-braiding-pultrusion, and laser spinning allow the transfer of many outstanding properties of biological models to biomimetic materials systems at reasonable costs. These methods enable the production of complex materials systems from small to large, combining a wide range of hierarchical levels (analogous to living nature). Additionally, an increased functionalization of the interfaces found in biological models could lead to the production of even more sophisticated materials systems, further advancing soft robots and soft machines.3
The development of plant-inspired soft robots and soft machines based on the form–structure–function relationships of biological models raises the questions of whether plants use embedded energy and have an embedded intelligence, two concepts widely discussed in soft robotics. Embedded energy definitively exists in many motile plant structures, as established nearly two decades ago by the physical considerations of Mahadevan and colleagues with regard to the necessity of speed boosts for (ultra-) fast plant movements.88,89 Virtually all fast plant movements rely on the release of stored or embodied elastic energy. Prime examples are the fast active traps in carnivorous plants (e.g., Reference 71), the “explosive” seed dispersal mechanisms as found in Hura crepitans, Impatiens sp., or Hamamelis sp.,90 and the spore release structures of some ferns and mosses (e.g., References 91 and 92). The question of the existence of embodied intelligence in plants, however, is not so easy to answer.
Embodied intelligence ... is the intelligent behavior in embodied and situated agents through ... the strict coupling between the agent and its environment (situatedness), mediated by the constraints of the agent’s own body, perceptual and motor system (embodiment).
[Citation from Cangelosi et al.93]
A definition of embodied intelligence from the Handbook of Computational Intelligence93 (but omitting the parts specific for computers and brains) suggests that embodied intelligence can also be attributed to plants. Embodied intelligence in plants is encoded in the structural setup of their motile materials systems, which have evolved an ability to react to environmental stimuli in a way that is advantageous to the survival of the plants. Examples include the environmentally triggered opening and closing movements of conifer cones and other plant seed pods, the active trapping mechanisms in carnivorous plants, and some self-sealing processes. Because all of these processes depend on the structural setup and the physical properties of the materials systems in the plant organs that react to the environmental physical stimuli (e.g., humidity, light, touch…), the term physical intelligence seems more appropriate94 than that of embodied intelligence. Indeed, complex plant structures, which consist of many individual motile elements (e.g., pine cones), show an even higher and much more complex functional integration. The orchestrated, entirely material-based opening of some pine cones, in which the helically arranged cone scales open one after another from cone base to top, can even be seen as a kind of physical swarm intelligence that is encoded in the materials system of the scales. By this the release of seeds is ensured, as the successive and orchestrated movement of the scales avoids mutual blockage of the scales (and, concomitantly, a prevention of seed release), which could occur if the latter opened randomly. Orchestrated opening is especially found in pine species with very dense scale arrangement and cone structure (e.g., Pinus jeffreyi), whereas in species with less dense scale arrangement in which no mutual blocking can occur (e.g., Pinus nigra), no or only rudimentary orchestration occurs. This orchestrated system alone may provide manifold inspiration for future technical applications.
For the presented plant-inspired soft machines and structures for facade shading and medical applications, we have focused on short-term movements of plants and plant organs that typically occur on a time scale between milliseconds and hours. For assessing the full potential plants for bioinspired research also long-term adaptations of plant as, for example, growth processes in woody plants should be considered. Additionally, field experiments will contribute to a better understanding of the complex interplay of (uncontrolled) natural environmental stimuli and the reactions of plants to them. Nevertheless, experiments under (semi-)controlled conditions, as possible in the Botanic Garden greenhouses, or under fully controlled conditions, as in phytochambers, are indispensable for (first) analyses of the stimulus–response chain and for a quantitative analysis of the form–structure–function coupling under controlled stimuli, which is the basis for all biomimetic transfer.
The above considerations clearly demonstrate that (bioinspired) materials research is a key technology for the next generation of soft robots and soft machines and is, in our opinion, extremely important for the further improvement and miniaturization of intelligent control systems.
Data availability
Data are available in the respective publications the review is based on and upon request.
References
S. Poppinga, T. Masselter, T. Speck, BioEssays 35, 649 (2013). https://doi.org/10.1002/bies.201200175
J.W. Kadereit, C. Körner, P. Nick, U. Sonnewald, Strasburger − Lehrbuch der Pflanzenwissenschaften (Springer, Cham, 2021)
B. Mazzolai, A. Mondini, E. Del Dottore, L. Margheri, F. Carpi, K. Suzumori, M. Cianchetti, T. Speck, S.K. Smoukov, I. Burgert, T. Keplinger, G. De Freitas Siqueira, F. Vanneste, O. Goury, C. Duriez, T. Nanayakkara, B. Vanderborght, J. Brancart, S. Terryn, S.I. Rich, R. Liu, K. Fukuda, T. Someya, M. Calisti, C. Laschi, W. Sun, G. Wang, L. Wen, R. Baines, S.K. Patiballa, R. Kramer-Bottiglio, D. Rus, P. Fischer, F.C. Simmel, A. Lendlein, Multifunct. Mater. 5(3), 032001 (2022). https://doi.org/10.1088/2399-7532/ac4c95
T. Speck, S. Poppinga, O. Speck, F. Tauber, Anthr. Rev. 9(2), 237 (2022). https://doi.org/10.1177/20530196211039275
P.T. Martone, M. Boller, I. Burgert, J. Dumais, J. Edwards, K. Mach, N. Rowe, M. Rueggeberg, R. Seidel, T. Speck, Integr. Comp. Biol. 50(5), 888 (2010). https://doi.org/10.1093/icb/icq122
B. Mazzolai, I. Walker, T. Speck (eds.), Generation GrowBots: Materials, Mechanisms, and Biomimetic Design for Growing Robots. Frontiers Research Topics. Frontiers in Robotics and AI (Frontiers Media SA, Lausanne, 2021). https://doi.org/10.3389/978-2-88971-185-7
X. Qian, Y. Zhao, Y. Alsaid, X. Wang, M. Hua, T. Galy, H. Gopalakrishna, Y. Yang, J. Cui, N. Liu, M. Marszewski, L. Pilon, H. Jiang, X. He, Nat. Nanotechnol. 14, 1048 (2019). https://doi.org/10.1038/s41565-019-0562-3
S.D. Cezan, H.T. Baytekin, B. Baytekin, Soft Robot. 7(4), 444 (2020). https://doi.org/10.1089/soro.2019.0036
U.G.K. Wegst, H. Bai, E. Saiz, A.P. Tomsia, R.O. Ritchie, Nat. Mater. 14, 23 (2015). https://doi.org/10.1038/nmat4089
R. Sachse, A. Westermeier, M. Mylo, J. Nadasdi, M. Bischoff, T. Speck, S. Poppinga, Proc. Natl. Acad. Sci. U.S.A. 117(27), 16035 (2020). https://doi.org/10.1073/pnas.2002707117
G.M. Durak, R. Thierer, R. Sachse, M. Bischoff, T. Speck, S. Poppinga, Adv. Sci. 9(22), 2201362 (2022). https://doi.org/10.1002/advs.202201362
I. Burgert, P. Fratzl, Integr. Comp. Biol. 49(1), 69 (2009). https://doi.org/10.1093/icb/icp026
R. Elbaum, in Plant Biomechanics, ed. by A. Geitmann, J. Gril (Springer, Cham, 2018), pp. 235–246
R. Elbaum, Y. Abraham, Plant Sci. 223, 124 (2014). https://doi.org/10.1016/j.plantsci.2014.03.014
C.J. Eger, M. Horstmann, S. Poppinga, R. Sachse, R. Thierer, N. Nestle, B. Bruchmann, T. Speck, M. Bischoff, J. Rühe, Adv. Sci. 9(20), 2200458 (2022). https://doi.org/10.1002/advs.202200458
C. Dawson, J. F.V. Vincent, A.-M. Rocca, Nature 390, 668 (1997). https://doi.org/10.1038/37745
E. Reyssat, L. Mahadevan, J. R. Soc. Interface 6(39), 951 (2009). https://doi.org/10.1098/rsif.2009.0184
F. Zhang, M. Yang, X. Xu, X. Liu, H. Liu, L. Jiang, S. Wang, Nat. Mater. (2022). https://doi.org/10.1038/s41563-022-01391-2
Y. Tahouni, F. Krüger, S. Poppinga, D. Wood, M. Pfaff, J. Rühe, T. Speck, A. Menges, Bioinsp. Biomim. (2021). https://doi.org/10.1088/1748-3190/ac0c8e
S. Poppinga, N. Nestle, A. Šandor, B. Reible, T. Masselter, B. Bruchmann, T. Speck, Sci. Rep. (2017). https://doi.org/10.1038/srep40302
D. Correa, S. Poppinga, M.D. Mylo, A.S. Westermeier, B. Bruchmann, A. Menges, T. Speck, Philos. Trans. R. Soc. A (2020). https://doi.org/10.1098/rsta.2019.0445
M. Rüggeberg, I. Burgert, PLoS ONE (2015). https://doi.org/10.1371/journal.pone.0120718
A. Le Duigou, T. Fruleux, R. Matsuzaki, G. Chabaud, M. Ueda, M. Castro, Mater. Des. (2021). https://doi.org/10.1016/j.matdes.2021.110158
S. Poppinga, C. Zollfrank, O. Prucker, J. Rühe, A. Menges, T. Cheng, T. Speck, Adv. Mat. 30(19), 1703653 (2018). https://doi.org/10.1002/adma.201703653
X. Qian, Y. Zhao, Y. Alsaid, X. Wang, M. Hua, T. Galy, H. Gopalakrishna, Y. Yang, J. Cui, N. Liu, M. Marszewski, L. Pilon, H. Jiang, X. He, Nat. Nanotechnol. 14, 1048 (2019). https://doi.org/10.1038/s41565-019-0562-3
N.P. Rowe, T. Speck, in The Ecology of Lianas, ed. by S. Schnitzer, F. Bongers, R. Burnham, F. Putz (Wiley-Blackwell, Chichester, 2015), pp. 323–341. https://doi.org/10.1002/9781118392409.ch23
J. Gallentine, M.B. Wooten, M. Thielen, I.D. Walker, T. Speck, K.J. Niklas, Front. Robot. AI 7, 118 (2020). https://doi.org/10.3389/frobt.2020.00118
S. Isnard, A.R. Cobb, N.M. Holbrook, M. Zwieniecki, J. Dumais, Proc. R. Soc. B 26(1667), 2643 (2009). https://doi.org/10.1098/rspb.2009.0380
B. Melzer, T. Steinbrecher, R. Seidel, O. Kraft, R. Schwaiger, T. Speck, J. R. Soc. Interface 7(50), 1383 (2010). https://doi.org/10.1098/rsif.2010.0140
P. Soffiatti, N.P. Rowe, Front. Robot. AI 7, 64 (2020). https://doi.org/10.3389/frobt.2020.00064
F. Klimm, S. Schmier, H.F. Bohn, S. Kleiser, M. Thielen, T. Speck, J. Exp. Bot. 73(4), 1190 (2022). https://doi.org/10.1093/jxb/erab456
T. Steinbrecher, E. Danninger, D. Harder, T. Speck, O. Kraft, R. Schwaiger, Acta Biomater. 6(4), 1497 (2010). https://doi.org/10.1016/j.actbio.2009.10.003
M.J. Jaffe, A.W. Galston, Annu. Rev. Plant Physiol. 19, 417 (1968). https://doi.org/10.1146/annurev.pp.19.060168.002221
F.E. Putz, N.M. Holbrook, in The Biology of Vines, ed. by F.E. Putz, H.A. Mooney (Cambridge University Press, Cambridge, 1991), pp. 73–97
C. Darwin, The Movements and Habits of Climbing Plants (John Murray, London, 1875)
D.T. MacDougal, Ann. Bot. os-10(3), 373 (1896). https://doi.org/10.1093/oxfordjournals.aob.a088619
W.D. Brush, Bot. Gaz. 53(6), 453 (1912). https://doi.org/10.1086/330845
R. Vidoni, T. Mimmo, C. Pandolfi, J. Bionic Eng. 12, 250 (2015). https://doi.org/10.1016/S1672-6529(14)60117-7
I. Must, E. Sinibaldi, B. Mazzolai, Nat. Commun. 10, 344 (2019). https://doi.org/10.1038/s41467-018-08173-y
F. Meder, S.P. Murali Babu, B. Mazzolai, IEEE Robot. Autom. Lett. 7(2), 5191 (2022). https://doi.org/10.1109/LRA.2022.3153713
L. Cortese, S. Milanovic, R. Vidoni, Appl. Bionics Biomech. 2017, 6450949 (2017). https://doi.org/10.1155/2017/6450949
Y. Cheng, R. Wang, K.H. Chan, X. Lu, J. Sun, G.W. Ho, ACS Nano 12(4), 3898 (2018). https://doi.org/10.1021/acsnano.8b01372
W. Hu, G. Alici, Soft Robot. 7(3), 267 (2020). https://doi.org/10.1089/soro.2019.0015
M. Kanik, S. Orguc, G. Varnavides, J. Kim, T. Benavides, D. Gonzalez, T. Akintilo, C.C. Tasan, A.P. Chandrakasan, Y. Fink, P. Anikeeva, Science 365(6449), 145 (2019). https://doi.org/10.1126/science.aaw2502
M.J. Jaffe, A.W. Galston, Plant Physiol. 41(6), 1014 (1966). https://doi.org/10.1104/pp.41.6.1014
A.J. Bowling, K.C. Vaughn, Am. J. Bot. 96(4), 719 (2009). https://doi.org/10.3732/ajb.0800373
S.J. Gerbode, J.R. Puzey, A.G. McCormick, L. Mahadevan, Science 337(6098), 1087 (2012). https://doi.org/10.1126/science.1223304
A. Goriely, M. Tabor, Phys. Rev. Lett. 80(7), 1564 (1998). https://doi.org/10.1103/PhysRevLett.80.1564
T. McMillen, A. Goriely, J. Nonlinear Sci. 12, 241 (2002). https://doi.org/10.1007/s00332-002-0493-1
H. Wu, Y. Zheng, Y. Zeng, Ind. Eng. Chem. Res. 54(3), 987 (2015). https://doi.org/10.1021/ie504305s
P.E.S. Silva, F. Vistulo de Abreu, M.H. Godinho, Soft Matter 13(38), 6678 (2017). https://doi.org/10.1039/C7SM01280B
M. Farhan, F. Klimm, M. Thielen, A. Rešetič, A. Bastola, M. Behl, T. Speck, A. Lendlein (submitted)
S. Tibbits, Archit. Des. 84(1), 116 (2014). https://doi.org/10.1002/ad.1710
D. Correa, A. Papadopoulou, C. Guberan, N. Jhaveri, S. Reichert, A. Menges, S. Tibbits, 3D Print. Addit. Manuf. 2(3), 106 (2015). https://doi.org/10.1089/3dp.2015.0022
T. Cheng, Y. Tahouni, D. Wood, B. Stolz, R. Mülhaupt, A. Menges, “Multifunctional Mesostructures: Design and Material Programming for 4D-Printing,” in Proceedings of the 5th Annual Symposium on Computational Fabrication (SCF '20) (Association for Computing Machinery, New York, 2020), pp. 1–10. https://doi.org/10.1145/3424630.3425418
T. Cheng, M. Thielen, S. Poppinga, Y. Tahouni, D. Wood, T. Steinberg, A. Menges, T. Speck, Adv. Sci. 8(13), 2100411 (2021). https://doi.org/10.1002/advs.202100411
United Nations Environment Programme (UNEP), 2020 Global Status Report for Buildings and Construction: Towards a Zero-Emission, Efficient and Resilient Buildings and Construction Sector - Executive Summary (2020). https://wedocs.unep.org/20.500.11822/34572
S. Reichert, A. Menges, D. Correa, Comput. Aided Des. 60, 50 (2015). https://doi.org/10.1016/j.cad.2014.02.010
J.M. Alvarez, J.F. Rocha, S.R. Machado, Braz. Arch. Biol. Technol. 51(1), 113 (2008). https://doi.org/10.1590/S1516-89132008000100014
M.-N. Grigore, C. Toma, Anatomical Adaptations of Halophytes. A Review of Classic Literature and Recent Findings (Springer, Cham, 2017), pp. 325–338. https://doi.org/10.1007/978-3-319-66480-4_8
A. Mader, M. Lange, J. Knippers, O. Speck, J. R. Soc. Interface 17(169), 20200358 (2020). https://doi.org/10.1098/rsif.2020.0358
S. Matschi, M.F. Vasquez, R. Bourgault, P. Steinbach, J. Chamness, N. Kaczmar, M.A. Gore, I. Molina, L.G. Smith, Plant Direct 4(10), e00282 (2020). https://doi.org/10.1002/pld3.282
B. Moulia, Biomimetics 2(3), 267 (1995)
B. Moulia, J. Plant Growth Regul. 19, 19 (2000). https://doi.org/10.1007/s003440000004
A. Körner, L. Born, A. Mader, R. Sachse, S. Saffarian, A.S. Westermeier, S. Poppinga, M. Bischoff, G.T. Gresser, M. Milwich, T. Speck, J. Knippers, Smart Mater. Struct. 27(1), 017001 (2017). https://doi.org/10.1088/1361-665X/aa9c2f
S. Schleicher, J. Lienhard, S. Poppinga, T. Speck, J. Knippers, Comput. Aided Des. 60, 105 (2015). https://doi.org/10.1016/j.cad.2014.01.005
F.J. Esser, P. Auth, T. Speck, Front. Robot. AI 7, 75 (2020). https://doi.org/10.3389/frobt.2020.00075
D. Hodick, A. Sievers, Planta 179, 32 (1989). https://doi.org/10.1007/BF00395768
F. Esser, F.D. Scherag, S. Poppinga, A. Westermeier, M.D. Mylo, T. Kampowski, G. Bold, J. Rühe, T. Speck, in Biomimetic and Biohybrid Systems. Living Machines, ed. by U. Martinez-Hernandez, V. Vouloutsi, A. Mura, M. Mangan, M. Asada, T.J. Prescott, P.F.M.J. Verschure (Springer, Cham, 2019), pp.114–121. https://doi.org/10.1007/978-3-030-24741-6_10
Y. Forterre, J.M. Skotheim, J. Dumais, L. Mahadevan, Nature 433, 421 (2005). https://doi.org/10.1038/nature03185
S. Poppinga, U. Bauer, T. Speck, A.G. Volkov, in Carnivorous Plants: Physiology, Ecology, and Evolution, ed. by A. Ellison, L. Adamec (Oxford University Press, Oxford, 2018), pp. 180–193. https://doi.org/10.1093/oso/9780198779841.003.0014
S. Poppinga, M. Joyeux, Phys. Rev. E 84, 041928 (2011). https://doi.org/10.1103/PhysRevE.84.041928
F.J. Tauber, P. Auth, J. Teichmann, F.D. Scherag, T. Speck, Biomimetics 7(3), 99 (2022). https://doi.org/10.3390/biomimetics7030099
A.S. Westermeier, R. Sachse, S. Poppinga, P. Vögele, L. Adamec, T. Speck, M. Bischoff, Proc. Biol. Sci. 285(1878), 20180012 (2018). https://doi.org/10.1098/rspb.2018.0012
Y. Lin, C. Zhang, W. Tang, Z. Jiao, J. Wang, W. Wang, Y. Zhong, P. Zhu, Y. Hu, H. Yang, J. Zou, Adv. Sci. 8(21), 2102539 (2021). https://doi.org/10.1002/advs.202102539
B. Ma, C. Xu, L. Cui, C. Zhao, H. Liu, ACS Appl. Mater. Interfaces 13(4), 5574 (2021). https://doi.org/10.1021/acsami.0c20418
K.S. Riley, K.J. Ang, K.A. Martin, W.K. Chan, J.A. Faber, A.F. Arrieta, Mater. Des. 194, 108888 (2020). https://doi.org/10.1016/j.matdes.2020.108888
F.J. Tauber, L. Riechert, J. Teichmann, N. Poovathody, U. Jonas, S. Schiller, T. Speck, in Biomimetic and Biohybrid Systems. Living Machines, ed. by A. Hunt, V. Vouloutsi, A. Mura (Springer, Cham, 2022), pp. 1–12
M.J. Jaffe, Plant Physiol. 51(1), 17 (1973). https://doi.org/10.1104/pp.51.1.17
J. Ou, Z. Ma, J. Peters, S. Dai, N. Vlavianos, H. Ishii, Comput. Graph. 75, 72 (2018). https://doi.org/10.1016/j.cag.2018.06.003
R.E. Ritzmann, R.D. Quinn, M.S. Fischer, Arthropod Struct. Dev. 33(3), 361 (2004). https://doi.org/10.1016/j.asd.2004.05.001
A.A. Sequeira, A. Usman, M.Z. Ali, O.P. Tharakan, Int. J. Autom. Mechatron. Robot. 3(1), 108 (2016)
C. Laschi, J. Rossiter, F. Iida, M. Cianchetti, L. Margheri (eds.), Soft Robotics: Trends, Applications and Challenges (Springer, Cham, 2017)
C. Majidi, Adv. Mater. Technol. 4(2), 1800477 (2018). https://doi.org/10.1002/admt.201800477
B. Mazzolai, A. Mondini, E. Del Dottore, in Mechanically Responsive Materials for Soft Robotics, ed. by H. Koshima (Wiley-VCH, Weinheim, 2020), pp. 363–394. https://doi.org/10.1002/9783527822201.ch15
A. Sadeghi, E. Del Dottore, A. Mondini, B. Mazzolai, Soft Robot. 7(1), 85 (2020). https://doi.org/10.1089/soro.2019.0025
F. Meder, M. Thielen, A. Mondini, T. Speck, B. Mazzolai, Energy Technol. 8(7), 2000236 (2020). https://doi.org/10.1002/ente.202000236
J.M. Skotheim, L. Mahadevan, Science 308(5726), 1308 (2005). https://doi.org/10.1126/science.1107976
J. Dumais, Y. Forterre, Annu. Rev. Fluid Mech. 44, 453 (2012). https://doi.org/10.1146/annurev-fluid-120710-101200
S. Poppinga, A.-S. Böse, R. Seidel, L. Hesse, J. Leupold, S. Caliaro, T. Speck, J. R. Soc. Interface 16(157), 20190327 (2019). https://doi.org/10.1098/rsif.2019.0327
S. Poppinga, T. Haushahn, M. Warnke, T. Masselter, T. Speck, PLoS One 10(10), e0138495 (2015). https://doi.org/10.1371/journal.pone.0138495
F. Gallenmüller, M. Langer, S. Poppinga, H.-H. Kassemeyer, T. Speck, AoB Plants 10(1), plx075 (2018). https://doi.org/10.1093/aobpla/plx075
A. Cangelosi, J. Bongard, N.H. Fischer, S. Nolfi, in Handbook of Computational Intelligence, ed. by J. Kacprzyk, W. Pedrycz (Springer, Berlin, 2015), pp. 697–714. https://doi.org/10.1007/978-3-662-43505-2_37
M. Sitti, Extreme Mech. Lett. 46, 101340 (2021). https://doi.org/10.1016/j.eml.2021.101340
Acknowledgments
This work was funded by the Ministry of Science, Research and the Arts of the Federal State of Baden-Wuerttemberg in the framework of the collaborative project “Bio-inspirierte elastische Materialsysteme und Verbundkomponenten für nachhaltiges Bauen im 21ten Jahrhundert” (BioElast) within the “Zukunftsoffensive IV Innovation und Exzellenz—Aufbau und Stärkung der Forschungsinfrastruktur im Bereich der Mikro- und Nanotechnologie sowie der neuen Materialien,” and by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) under Germany’s Excellence Strategy in the Clusters of Excellence IntCDC at the University of Stuttgart [EXC 2120/1—390831618] and livMatS @ FIT at the University of Freiburg [EXC 2193/1—390951807]. Additional funding was received from the European Union’s Horizon 2020 Research and Innovation Programme under Grant Agreement No. 824074 (GrowBot).
Funding
Open access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Speck, T., Cheng, T., Klimm, F. et al. Plants as inspiration for material-based sensing and actuation in soft robots and machines. MRS Bulletin 48, 730–745 (2023). https://doi.org/10.1557/s43577-022-00470-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/s43577-022-00470-8