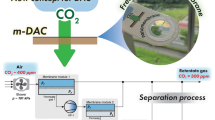
Abstract
Reducing CO2 emissions alone will not suppress global warming, and it is necessary to capture the CO2 that has been cumulatively emitted into the atmosphere as well. For this reason, negative CO2 emission technology, a technology to capture CO2 from the atmosphere, is considered essential. Especially, direct capture of CO2 from the air, so-called direct air capture (DAC) has attracted much attention as one of promising technologies, because of the high potential capacity of CO2 capture. In general, absorption, adsorption, and membrane separation are known as representative CO2 capture technologies, and DAC is basically based on these technologies. In particular, DAC using absorption and adsorption methods has already reached the level of plant scale, but the desorption process of captured CO2 from the absorbent or adsorbent consumes a large amount of heating energy and water. On the other hand, membrane separation is generally considered as a most cost- and energy-efficient process among these capture technologies, but DAC by membrane separation has not been considered at all due to the immaturity of the membrane performance for CO2 capture, especially CO2 permeance. However, recent developments in membrane technology have brought the possibility that membrane processes can be considered as a new approach to DAC. In this article, the potential of membrane technologies as DAC is discussed and future technology target is proposed.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Currently, various climate change-related activities are being carried out all over the world to realize a “decarbonized society,” and R&D of related technologies is also being vigorously promoted. In particular, the reduction of carbon dioxide (CO2) emissions has become an urgent issue. Development of carbon capture and storage (CCS) technology is being promoted to reduce CO2 emissions as dealing with large-scale CO2 emission sites such as thermal power plants. At the same time, CO2 emissions reduction alone has proven to be insufficient to reduce the concentration of CO2 in the atmosphere, so it is essential to also implement technologies of direct air capture (DAC) to capture the CO2 already released into the atmosphere.1
As a source of CO2 capture, it is important to compare the features of the atmosphere with those of large-scale emission sources such as thermal power plants in the development of DAC technology. First of all, CO2 concentration of the air is ultrasmall (about 400 ppm) compared to that of the flue gas emitted from plants and factories (>10%). This low CO2 concentration is causing technical difficulties. Second is an amount issue. The amount of air needed to be processed is enormous. Third, the atmosphere exists everywhere on the planet, but large CO2 emission sites such as thermal power plants are located in specific places. Based on these characteristics of air, DAC technology should possess the ability to process a huge amount of air efficiently and should be location-independent.
In terms of mass treatment, liquid absorption is considered the main capture technology because of its large process capacity.2 However, a sorbent-based process generally requires a specific site where a capture plant is installed. Considering the ubiquitous nature of the atmosphere, this adsorbent process may not be well suited, because it generally requires large-scale, low-cost green energy to recover CO2 from absorbent, water, and harzardous chemicals and these factors often limit an installation site. In particular, 78% of the world's CO2 emissions come from G20 member countries.3 Given the higher concentration of CO2 in the atmosphere around major urban cities, CO2 capture is particularly important in these areas where population and economic activities are concentrated. In such urban areas, distributed deployment of smaller systems is more suited because the space is limited. Therefore, the new concept of “ubiquitous CO2 capture,” in which atmospheric CO2 can be captured anywhere, becomes increasingly valuable. This concept is similar to the photovoltaic (PV) system in electricity generation. Sunlight falls universally on the Earth and conventional PV systems can capture sunlight in any location, from small to large, if space is available for installation. One can find a common nature in electric power generation and DAC. A thermal power plant is a centralized system to produce a large amount of electricity efficiently, but the installation site is limited, whereas PV is a decentralized one. In DAC, technologies to fit both large-centralized and small-distributed systems are necessary.
For a distributed and scalable DAC system such as PV systems, it is necessary to develop a CO2 capture technology that can be modularized without hazardous chemicals and requires low energy and a small footprint. Although many approaches have been reported so far, CO2 capture by membranes requires less energy and space and can be adjusted to any scale by combining membrane units. Therefore, from the viewpoint of ubiquitous CO2 capture, membrane separation becomes highly advantageous.
Possibilities of membrane-based DAC (m-DAC)
Generally, it has been believed that capturing CO2 directly from the air by membranes is impractical because the CO2 concentration in the air is too small (400 ppm) to give a sufficient driving force for CO2 permeation through the membranes.4 In addition, conventional membranes have a low CO2 permeance and thus require energy to set a large pressure difference or a tremendous membrane area for DAC use. Conversely, if the CO2 permeance of membranes is high enough, membrane-based DAC will become a promising technology.
To explore the potential of m-DAC, we carefully examined membrane system performance for capturing CO2 from the atmosphere by using process simulation.5 Multistage separation was considered to achieve practical preconcentration because single-step separation is insufficient to obtain higher CO2 concentration.6 Figure 1 shows a schematic diagram of the membrane separation process. Compared to industrial separation processes, a more straightforward design of membrane stages with the same separation performance connected in series was employed. The concentration of CO2 in the retentate gas of each separation step was set to 300 ppm. As such, the total residue of the system could be seen as having an average CO2 concentration similar to the preindustrial atmospheric level. We have also optimized the separation system to ensure that CO2 concentration in the final permeate gas would be enriched by a factor of 1000 or more (>40 mol% of CO2 in a product). Based on this scenario, a membrane with CO2 permeance of 10,000 GPU (1 GPU = 7.5 × 10–12 m3(STP) m−2 s−1 Pa−1, STP: standard temperature and pressure) and a CO2 selectivity of 40 for other gases could capture 1 kg of CO2 per day with a total membrane area of less than 5 m2.
Considering the conventional membrane module geometries, the membrane with an area of 5 m2 can compactly fit into a volume of fewer than 1000 cm3.7 With such small membrane module sizes, membrane-based CO2 capture units can be installed almost anywhere.
In the separation process described above, most of the energy is used by vacuum pumps at the permeate side of each membrane. Figure 2 summarizes the performance of the four-stage separation system depending on the concentration of CO2 in the surrounding atmosphere. Figure 2a shows that four stages are essential to achieve the relevant concentration of the CO2 in the product, as only the tiny preconcentration can be performed in one step (independent of membrane separation properties if realistic vacuum pumps are considered). The first step is the most energy-intensive because the system should process a large amount of air (takes >80% of total energy expenditure). Although requiring lots of energy for the preconcentration from ambient air, it drops significantly when the feed concentration increases. Moreover, this result is obtained under specific conditions, and the total performance of membrane separation is affected by many operational parameters, such as gas selectivity, pressures in the system, and stage-cut, in addition to gas permeance. Further parametric analysis of separation performance may provide the pathway how to reduce the energy consumed in the m-DAC process.
Influence of CO2 content in a surrounding atmosphere (feed gas) on (a) the CO2 concentration in the product gas at each separation step, (b) the energy required to capture 1 kg of CO2 per day (blue circles), and relative contribution of each stage in total energy (grayscale circles) of the four-stage membrane separation system. Membrane properties used in simulation assumed the permeance of CO2 of 10,000 GPU, selectivity toward other gases in the system of 40, permeate pressures of 2 kPa used at each separation stage resulting in the feed to the permeate pressure ratios of ~55.
Generally speaking, the average CO2 concentration in the air is around 400 ppm. However, there are many sites in human environment where it is significantly higher than that. For example, the average CO2 concentration in office spaces reaches nearly 1000 ppm8 and, thus, office buildings would be good candidates as small-scale CO2 capture sites. Figure 2b shows the relationship between the concentration of CO2 in the air and the energy required for pumps to generate transmembrane pressure differences. As can be seen, higher CO2 concentration in the feed dramatically reduces the energy required for CO2 capture. At the same time, the product purity also increases. For example, the Roppongi Hills Mori Tower Building in Tokyo has an office area of 179,013 m2, and if the ceiling height is set at 3 m, the volume of the office space is 537,039 m3. Considering that the average CO2 concentration in the office during the daytime is about 600 ppm and that the air conditioning system ventilates several times in an hour (about 5 times), the weight of CO2 in this exhausted air is about 17,000 tons/year.9 If, for example, 25% of this CO2 is recovered, 4200 tons of CO2 can be collected from just one building. This potential is not negligible. Similarly, schools, shopping malls, and places where people gather should be potential sites to capture CO2. In addition, there are many candidate places where CO2 concentration is high in a city, and urban regions have great potential as CO2 capture sites.
Application scenarios for m-DAC in carbon capture, utilization, and storage
In conventional CO2 capture and storage (sequestration), CO2 capture sites are generally identified as thermal power plants, cement plants, and steel mills, which present a certain limitation for technology adoption. In addition, the gas purity target for geological storage of CO2 after capture is 90% or higher.10 Therefore, the required performance of CO2 capture systems is automatically set to meet this target condition. This is a single path that allows each process target to be set clearly and concisely because starting and ending points of CCS are identified. As one can realize, there are many opportunities and scenarios to deploy the anthropogenic CO2 capture as previously described. At the same time, it is also necessary to consider how to manage the CO2 after capturing because carbon capture, utilization, and storage (CCUS) is a continuous process. The m-DAC process can produce the CO2 streams with different gas compositions after separation, depending on the future use. In contrast, the absorbent-based process only offers highly pure CO2 gas (more than 90% in general). As described above, the required membrane performance largely depends on the subsequent process and demands. In general, there are three major routes to treat captured CO2 after DAC, as summarized in Figure 3. The membrane systems' versatility allows the design of the membrane process for DAC, considering the subsequent CO2 treatment after capture.
Utilization of CO2
Direct use of CO2
In this case, captured CO2 is used without any conversion. CO2 is often used for welding, dry ice, soda, and so on, and high CO2 concentration is generally preferred. However, low concentrations of CO2 gas are sometimes effective as well. One example is CO2 supplementation on vegetable farming in greenhouses. The CO2 concentration in the supply gas is not high, only in the range of a few thousand ppm. This concentration may be easily achievable by a single step of the m-DAC process. In this case, the m-DAC process is advantageous because it can continuously supply an enriched CO2 stream without post-treatment after CO2 capture as in the absorbent-based process. In same context, CO2 supply to microalgae cultures would be effective to enrich the production of biomass and biofuels.
CO2 recycling
CO2 can be considered as a useful carbon source alternatively to fossil resources. CO2 conversion to value-added compounds, including fuel, commodity chemicals, and other carbon-based compounds plays an important role for CO2 recycling. Generally, a highly pure CO2 stream is preferred to convert it chemically because high concentration CO2 enhances reactivity and efficiency of the conversion reaction. But production of highly pure CO2 gas is often energy-intensive. In terms of the total amount of energy used from CO2 capture to conversion, in some cases, chemical conversion using low concentrations of CO2 can also be effective, when conversion reaction proceeds effectively. For example, there are some reports on the chemical conversion of CO2 by using low-concentration CO2.11,12,13 Very recently, CO2 conversion to CH4 with low CO2 concentration gas (100–400 ppm) was successfully achieved, though it requires rather high temperature and pressurized condition.
CO2 storage
DAC-combined with CO2 storage (DACCS) is also important to reduce CO2 concentration in the air permanently. Generally, geological storage of CO2 requires highly pure CO2 gas (more than 98%). Although m-DAC produces relatively low-purity CO2, the other gaseous components (mainly nitrogen and oxygen) are not hazardous. There may be an opportunity to inject low concentrations of CO2 into the subsurface, if the cost is acceptable. This is because the ubiquity of the atmosphere allows for CO2 capture and storage at remote sites such as deserts and ocean platforms in order to reduce the cost of transporting the captured CO2 and to ensure social acceptability of CO2 storage. We previously evaluated geological storage of the low-purity CO2 captured via m-DAC technology by calculating the density of CO2–N2–O2 mixtures via molecular dynamics simulation and evaluated the cost of the low-purity CO2 storage.14 Our evaluation suggests that the storage of low-purity CO2 in geological formations is environmentally acceptable and economically viable, although it needs to be stored in a slightly deeper place than high-purity CO2.
Overall, it is clear that a high concentration of CO2 gas is not always necessary for CO2 conversion and storage from the previous considerations. Thus, there are many routes to utilize and process the gas from m-DAC, which has a relatively low CO2 concentration compared to capture using an adsorbent.
It is important to emphasize that m-DAC is not the only approach, but is a complementary technology to conventional sorbent-based DAC. Sorbent-based DAC technologies can process a large amount of the air efficiently, though the installation site and size may be limited. The m-DAC can handle relatively small amounts of air, but due to the nature of the technology, it can be installed in large numbers, in a variety of locations. Thus, m-DAC opens new opportunities and concepts to capture CO2 from the air, “CO2 capture anywhere (ubiquitous CO2 capture),” when conventional DAC is hardly applicable.
Membrane design for DAC
Gas permeance
m-DAC requires high membrane performances, mainly on gas permeance and selectivity. Gas permeance of a membrane is generally inversely proportional to a membrane thickness. Therefore, the simplest approach to enhance gas flux through a membrane is a thinning of membrane.15 But, open space at the permeate side is necessary to release gas after membrane permeation. Therefore, the membrane should be self-sustaining, maintaining its own structure over the open pores on the support layer surface.
To avoid membrane breakage during membrane fabrication and gas separation, support films with smaller openings (less than 10 nm) are often used when the thickness of the membrane is thinner than the submicron scale. A gutter layer composed of highly gas-permeable materials such as poly[(1-trimethylsilyl)-1-propyne] (PTMSP)16 and poly(dimethylsiloxane) (PDMS)17 are often deposited onto a porous support for the formation of gas selective layers on it.18, 19 However, when the membrane material is coated directly on the porous substrate, polymeric materials inevitably penetrate into the open pores on the support surface, making it impossible to form a layer with a uniform thickness. Essentially, when the film thickness is identical, the permeability and selectivity should be the same (when the same support film is used). However, even if the PDMS layer fabricated directly on the support has the same reported thickness values, there are variations in the film performance. This is probably because the thickness of the entire film is not uniform, despite being locally the same (see the supporting information in Reference 8). The fabrication of thin membranes with stable CO2 permeation is still a central issue in membrane fabrication.
We have already reported the fabrication of free-standing nanomembranes with planar dimensions sufficiently large compared to the membrane thickness.20 In this case, a membrane transfer approach was employed. In detail, the gas separation membrane is first prepared on a sacrificial layer on a solid substrate by spin-coating. By dissolving the sacrificial layer, the membrane can be delaminated from the substrate, placed on the appropriate porous support and used practically for the gas separation (Figure 4). Despite the thickness in nanoscale range membrane can be free-standing with only perimeter support as shown in Figure 5.
This method allows for precise and reproducible control of the thickness of the membrane. Based on this approach, free-standing nanomembranes of poly(dimethylsiloxane) (PDMS) with controlled thickness were fabricated and transferred onto a porous support, and their gas permeability was investigated.19 A nanomembrane with a thickness of as thin as 34 nm was successfully prepared. The CO2/N2 selectivity of this film was about 11, which was almost identical to that of the thick film. In other words, despite the thickness of 34 nm, there were no defects leading to a gas leak. The CO2 permeance of this nanomembrane showed a very high value of ~40,000 GPU reproducibly. This is the world's highest CO2 permeance reported so far and exceeded the CO2 permeance required for membrane-based DAC as discussed previously. In addition, the PDMS membrane with a thickness of 150 nm captured 25% of CO2 from 1000 ppm CO2 feed mixed with N2. Thus, making the membrane thinner is an effective approach to increasing gas permeance. However, simple thinning is not sufficient for gas separation membranes because they need to have the mechanical strength to maintain the membrane structure even under the pressure difference between the permeation side and the supply side in order to permeate the gas. For this purpose, incorporation of nanofillers is effective to enhance mechanical strength of nanomembranes and there have been some report to prepare free-standing nanomembranes with the use of nanofiller components.21,22,23
Gas selectivity
As shown in Figure 6, the permeability of CO2 decreased as the thickness decreased below a few hundred nm.20 The arrangement and size of the pore apertures on the porous support surface and the ratio of membrane thickness to the pore size also affect the gas permeation behavior.24 However, one cannot conclude that these are all the reasons for reducing gas permeability. The CO2 permeance against thickness composes of two relationships with the transition region at 100–1000-nm thickness. In the thickness region of micron scale (region 1 in Figure 6), similar permeability was almost constant of about 3500 in the barrer unit (1 barrer = 7.5 × 10–12 m3 m m−2 s−1 Pa−1) that agrees with previously reported values for a PDMS membrane of 1-μm thickness.25, 26 In contrast, CO2 permeability decreases as the film thickness decreases in the submicron region (region 2 in Figure 6). Obviously, the gas permeation process follows two different mechanisms.
It has been accepted that gas permeability (P) in non-porous membranes is explained by the solution-diffusion model expressed in Equation 1.
where S and D are the solubility and diffusivity of gas, respectively. Based on this model, the whole membrane part is assumed to be uniform and is characterized by parameters of D and S. Constant gas permeability in the 1–10-μm-thickness region means that gas diffusion in a membrane governs gas permeance performance because the permeation rate is inversely proportional to a membrane thickness. On the other hand, a different relation holds between permeability and thickness in the nanometer region. The diffusion distance within the membrane interiors becomes small in the thinner membranes, and the influence of the diffusion process on the permeation kinetics may be less. In such a thickness region, the total permeation process appears to be largely governed by the kinetics of the solubilization process. In addition, the constant selectivity value observed for different membrane thicknesses suggests that the gas selectivity is not affected by the diffusion process in the membrane interior. Therefore, we conclude that the kinetics of permeation is different between the two parts of the membrane, the interior and the interface. The sorption/desorption rate of a CO2 molecule at the interfacial area is apparently independent from membrane thickness, therefore the migration of CO2 gas across the very thin membrane is largely determined in this sorption/desorption step at the membrane interface. When the membrane becomes thick, beyond one micrometer, gas diffusion in the membrane interior mostly governs the permeation rate. Further supporting, the CO2/N2 selectivity is independent of the membrane thickness for the whole thickness range. This result is justified by assuming that the gas selection proceeds only at the interfacial area. The membrane interior appears not influential for gas separation. This interpretation suggests the molecular design of a membrane surface plays an important role to determine the gas selectivity. Based on this idea, we prepared PDMS nanomembranes coated with a thin layer (2–20-nm thick) of a block copolymer (Pebax-1657) containing CO2-selective poly(ethylene glycol) chains.27 As a result, it was found that the introduction of the Pebax-PDMS interfacial layer with only a few nanometers thickness dramatically improves the CO2/N2 selectivity to about 70, although the gas permeance decreases. In this case, pretreatment of PDMS nanomembranes by oxygen plasma results in the reduction of gas permeance because a thin layer of oxide PDMS (i.e., SiO2) is formed on the PDMS layer for uniform coating of hydrophilic block copolymers. Thus, mild and thin modification of the nanomembrane surface will be the next challenge to develop a highly permeable and selective CO2 separation nanomembrane for m-DAC use.
In particular, it should be emphasized that CO2/O2 separation becomes important when the captured CO2 is used in chemical conversion processes, because oxygen prevents the efficient reduction of CO2.28 To our best knowledge, CO2/O2 separation by a membrane has rarely been addressed in the literature. All of CO2 capture technologies, including this m-DAC process, should be combined with successive processes to manage captured CO2. Chemical conversion is one of the major process to circulate CO2 as carbon source. Therefore, the product gas from the m-DAC system should not only achieve relevant concentration of CO2, but also significantly reduced concentrations of O2, which puts the requirement of high CO2/O2 selectivity, in addition to CO2/N2 selectivity, though such a separation situation has never been considered.
In summary, considering the material aspect, first, membranes for DAC require high gas permeance, not permeability. Higher permeance allows the development of size-feasible membranes for DAC. Second, CO2 selectivities play a very important role, as described earlier.5, 6 The upper bound limitation for organic polymers implies limited space to simultaneously explore membrane materials that satisfy both requirements.15 Therefore, membrane structure should be considered well. The thin-film composite membrane approach is one direction to seek better membranes for DAC, because ultrathin gas selective layers are separately designed on a highly gas-permeable gutter layer. This approach may provide a qualitatively new, rational way to explore new membrane materials for enhancing membrane performance.
Conclusion
Membrane-based DAC is an important addition to the portfolio of methods aimed to realize ubiquitous CO2 capture as well as solar power generation. To achieve this, the gas selectivity of the separation nanomembranes must be improved without decreasing CO2 permeances. If such membranes are developed, they can be assembled in DAC units and combined with a CO2 conversion system. This combined system can produce value-added carbon compounds by using renewable electricity and hydrogen from water electrolysis. These small systems would contribute to the establishment of a carbon-neutral society where carbon materials are produced on-site and consumed locally. Also, if low-purity CO2 can be safely contained underground, it can lead to the construction of a beyond zero-carbon society, further advancing the goal of net-zero CO2 emissions.
Data availability
All data generated and analyzed during this study are included in this published article.
References
J.C. Minx, W.F. Lamb, M.X. Callaghan, S. Fuss, J. Hilaire, F. Creutzig, T. Amann, T. Beringer, W. de Oliveira Garcia, J. Hartmann, T. Khanna, D. Lenzi, G. Luderer, G.F. Nemet, J. Rogelj, P. Smith, J.L. Vicente Vicente, J. Wilcox, M. del Mar Zamora Dominguez, Environ. Res. Lett. 13(6), 063001 (2018)
E.S. Sanz-Pérez, C.R. Murdock, S.A. Didas, C.W. Jones, Chem. Rev. 116, 11840 (2016)
United Nations Environment Programme (UNEP), Emissions Gap Report 2019. Executive Summary. (UNEP, Nairobi, 2019)
D.W. Keith, Science 325, 1654 (2009)
S. Fujikawa, R. Selyanchyn, T. Kunitake, Polym. J. 53, 111 (2021)
C. Castel, R. Bounaceur, E. Favre, Front. Chem. Eng. 3, 1 (2021)
D. Li, R. Wang, T.-S. Chung, Sep. Purif. Technol. 40, 15 (2004)
O.A. Seppanen, W.J. Fisk, M.J. Mendell, Indoor Air 9, 226 (1999)
R. Dittmeyer, M. Klumpp, P. Kant, G.A. Ozin, Nat. Commun. 10, 1818 (2019)
Todd, S. et al., Effects of Impurities on Geological Storage of CO2 (Report 2011/04, IEAGHG, 2011). https://ieaghg.org/docs/General_Docs/Reports/2011-04.pdf
B. Kim, S. Ma, H.-R. Molly Jhong, P.J.A. Kenis, Electrochim. Acta 166, 271 (2015)
H. Kumagai, T. Nishikawa, H. Koizumi, T. Yatsu, G. Sahara, Y. Yamazaki, Y. Tamaki, O. Ishitani, Chem. Sci. 10(6), 1597 (2019)
F. Kosaka, Y. Liu, S.-Y. Chen, T. Mochizuki, H. Takagi, A. Urakawa, K. Kuramoto, ACS Sustain. Chem. Eng. 9(9), 3452 (2021)
T. Tsuji, M. Sorai, M. Shiga, S. Fujikawa, T. Kunitake, Greenh. Gases Sci. Technol. 11, 610 (2021)
R. Selyanchyn, S. Fujikawa, Sci. Technol. Adv. Mater. 18, 816 (2017)
T. Li, Y. Pan, K.-V. Peinemann, Z. Lai, J. Memb. Sci. 425–426, 235 (2013)
W. Yave, A. Car, J. Wind, K.-V. Peinemann, Nanotechnology 21, 395301 (2010)
K.A. Lundy, I. Cabasso, Ind. Eng. Chem. Res. 28, 742 (1989)
R.W. Baker, B.T. Low, Macromolecules 47, 6999 (2014)
S. Fujikawa, M. Ariyoshi, R. Selyanchyn, T. Kunitake, Chem. Lett. 48, 1351 (2019)
A. Mersha, S. Fujikawa, ACS Appl. Polym. Mater. 1, 112 (2019)
Y. Wang, S. Lee, H. Wang, Z. Jiang, Y. Jimbo, C. Wang, B. Wang, J.J. Kim, M. Koizumi, T. Yokota, T. Someya, Proc. Natl. Acad. Sci. U.S.A. 118, e2111904118 (2021)
M. Ariyoshi, S. Fujikawa, T. Kunitake, ACS Appl. Mater. Interfaces 13, 61189 (2021)
M. Kattula, K. Ponnuru, L. Zhu, W. Jia, H. Lin, E.P. Furlani, Sci. Rep. 5, 15016 (2015)
T.C. Merkel, V.I. Bondar, K. Nagai, B.D. Freeman, I. Pinnau, J. Polym. Sci. B Polym. Phys. 38, 415 (2000)
M.J. Yoo, K.H. Kim, J.H. Lee, T.W. Kim, C.W. Chung, Y.H. Cho, H.B. Park J. Memb. Sci. 566, 336 (2018)
O. Selyanchyn, R. Selyanchyn, S. Fujikawa, ACS Appl. Mater. Interfaces 12, 33196 (2020)
K. Williams, N. Corbin, J. Zeng, N. Lazouski, D.-T. Yang, K. Manthiram, Sustain. Energy Fuels 3(5), 1225 (2019)
Acknowledgments
Authors acknowledge the funding of the “Moonshot Research and Development Program” (JPNP18016), commissioned by the New Energy and Industrial Technology Development Organization (NEDO). This work was supported by the World Premier International Research Center Initiative (WPI), sponsored by the Japanese Ministry of Education, Culture, Sports, Science, and Technology. S.F. acknowledges the Japan Society for the Promotion of Science (JSPS) for a Grant-in-Aid for Scientific Research (B) (JP20H02781). R.S. acknowledges the Japan Society for the Promotion of Science (JSPS) for a Grant-in-Aid for Early Career Scientists (JP19K15342).
Conflict of interest
The authors declare no competing interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fujikawa, S., Selyanchyn, R. Direct air capture by membranes. MRS Bulletin 47, 416–423 (2022). https://doi.org/10.1557/s43577-022-00313-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/s43577-022-00313-6