Abstract
Due to our increasing awareness of the impact of climate change on our society, unit operations in our manufacturing processes, including those in chemical industry, have to be greenified and made less dependent of fossil resources. This so-called electrification of the chemical industry is still yet in its infancy but there are many scientific and technological challenges to be solved. This article provides some directions for further research for scientists in both academia and industry to move step by step to an e-chemistree. These important but far from trivial energy and materials transitions require not only the introduction of new ways of heat management and other, often not yet fully explored, chemical conversion processes in which green electrons are used, but also the development of new materials including large-scale heating coils, easily chargeable battery systems as well as catalyst materials. For each of these developments, there is the issue of materials scarcity as well as durability as the introduction of these production processes should also be cost effective and overall more sustainable than the existing ones.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Average global temperatures have risen significantly since the industrial revolution, and the last decade was the warmest decade on record. Of the 18 warmest years, 17 have occurred since 2000. The majority of evidence as also recently stipulated in the Intergovernmental Panel on Climate Change (IPCC) indicates that this is due to the rise of greenhouse gas emissions (GHG) produced by human activity. Between 2000 and 2018, global GHG emissions increased on average by 2.4% every year.1 Over the same period, the direct industrial CO2 emissions increased even more, that is by about 3.7% every year.2 Therefore, next to the legally binding Paris Agreement, new initiatives are being launched, with the most ambitious one the “Fit for 55 package” set by the European Commission in 2021.3 The target of “Fit for 55” is to reduce GHG emissions by 55% by 2030 compared to 1990 levels, which is in chemical industry terms, basically doing this tomorrow. One can only imagine the panic in many board rooms to meet this binding target.
For the chemical industry where do these emissions come from? In chemical and petrochemical processes, greenhouse gases can be produced as a byproduct from the process itself—for example, CO2 can be emitted during the production of ammonia, which is used for purifying water supplies, cleaning products, and as a refrigerant, and used in the production of many materials including plastics, fertilizers, pesticides, and textiles. In 2016 this was 2.2% of all the global GHG emissions. However, chemical and petrochemical manufacturing also produces emissions from energy inputs—these related emissions are even higher, and were 3.6% of the global emissions in 2016. To reduce not only the direct GHG emissions but also the energy related ones governments are betting on the massive growth of the production of renewable electricity. This is nowadays coined the “electrification” of the process industry. Electrification of the chemical industry is defined as the use of electricity to drive chemical processes including conversion, separation, and purification, and providing the necessary materials and utilities to assist in operating and controlling such processes.4 However, the total electrification of the chemical industry is today more a pipedream than a reality.5 The main issues are as follows:
-
1.
There is not yet enough renewable electricity and in particular in the locations where chemicals are produced today.
-
2.
The chemical industry is often known to be not very agile. As investments are depreciated over decades, it is clear that the dominant processes that are in place today for large volume chemicals will be the same until at least 2030.
-
3.
Large-scale chemical processes can simply not be turned on and off, definitely not in a wink of an eye, but getting back on spec takes weeks.
-
4.
Chemical production, because of the high CAPEX, only makes sense when the plant is running 8000 h per year. Availability is a crucial plant metric next to profitability and energy efficiency.
Next to these challenges “safety” is always the number one priority as the chemical industry has in the eyes of the general public a bad reputation because of the numerous incidents over the past decades. Processes that have been developed over more than a century in some cases might have to be replaced by techniques that currently only have been proven on a lab scale or do not currently exist as we speak. One can imagine that all these things give CEOs and CTOs of major chemical companies headaches.
To make things even more complex and challenging, in several countries and in particular Europe, this electrification needs to be combined with defossilization. Defossilization is not the same as decarbonization6 but is enabling for the transition from a linear to a circular carbon economy. It is clear that decarbonization of the chemical industry strictly speaking is not realistic as most of the chemicals contain carbon. How to overcome the critical hurdle of developing cost-efficient low-carbon technologies to convert renewable energy and resources that are abundant, such as CO2, N2, H2O, into fossil-free fuels and base chemicals for industry and agriculture is another challenge that needs to be overcome. One option of how to get there is illustrated schematically in Figure 1, where in this approach, electricity provides the needed amount of energy to drive the given chemical reactions, such as for the production of ammonia. The electrochemical conversion of water (i.e., water electrolysis) produces oxygen and more importantly hydrogen gas, a key enabler for many electrification visions. By using renewable electricity sources, hydrogen production becomes free of carbon emissions. Beyond water electrolysis, also CO2 and water can be directly transformed into a chemical feedstock (e.g., CO and ethylene) via the co-electrolysis or direct CO2 electroreduction technologies. Again, it needs to be stressed that economically this scheme is not currently competitive with the best available conventional technologies, based on fossil resources.
But there is more than economics, given government interventions some of the process options could actually make sense. Although numerous challenges remain it is no longer a question if electrification of the chemical industry will happen, but when it will be the dominating technology. For sure, it will definitely come at a cost as the CAPEX’s for chemical complexes is at least several billion dollars7 and the lifetime of these facilities is expected to be greater than 30 years. Making sure that this transition can happen is the first step, because today the high electricity prices make electrification non-competitive. But in particular on the material side this brings new challenges, which could slow down electrification of the chemical industry substantially. How this transition could happen is elaborated in the following sections.
Transition to an electrified chemical industry: the e-chemistree
It is a priori clear that a completely electrified chemical industry will not happen overnight. Very likely there will be a long transition period, in which at first the structure of today’s chemical industry will be largely maintained, at least over a couple decades. As is shown in Figure 2 the structure of the chemical industry is typically presented in the form of a chemistree.8 The Chemistree is just a matter of common sense as it is synonymous for an intelligent integrated production system, with synergies that are often of critical importance for success. In other words, also in the future there only will be a few hundred major basic products and intermediates that will be produced on a scale of at least a few hundred thousand to several million tons per annum worldwide. Also in the e-chemistree, shown in Figure 2, this relatively small group of key products, which are in turn produced from a limited amount of raw materials, are the stable foundation on which the many branches of the chemical industry (dyes, pharmaceuticals, polymers, and coatings), with their many thousands of often only short-lived end products, are based. However, the raw materials will change, and as was also illustrated in Figure 1, they will move toward more sustainable alternatives, such as water, waste, biomass, air, biogas, and probably also (point source) CO2. This is in line with the fact that if one wants to make any sort of impact on the global CO2 emissions on short notice, the bottom of the tree needs to be tackled first because there the processes with the large emissions can be found. Note that also other trees have been proposed such as the “green chemistree”9 and the final outcome might be a mix of different trees depending on the particular region as well as on the government’s policies in a particular country or continent.
As illustrated in e-chemistree, the production of fuels and chemicals from green electricity, the so-called e-fuels and e-chemicals, such as ammonia, carbon monoxide, ethylene, and methanol, will likely be key elements of an electrified chemical industry. At first the production of these e-fuels or e-intermediates will be carried out in a two-step approach: first, the independent production of renewable power will be combined with the subsequent synthesis of intermediate products, such as green hydrogen, via water electrolysis. Second, these intermediate compounds serve as energy-rich building blocks for the production of renewable fuels and chemicals (e.g., through conventional conversion processes). These include the industrially well-developed Fischer–Tropsch Synthesis (FTS) process for the production of fuels for transportation from syngas (CO/H2 mixture) or the Haber–Bosch cycle for ammonia production from green hydrogen and nitrogen. Also less-industrially developed upgrading routes are promising such as taking methanol as a feedstock for drop-in fuels or the biological conversion of hydrogen and CO/CO2 to ethanol or methane.
On the other hand, it also possible to produce renewable fuels and chemicals directly from solar energy.10 This second approach takes inspiration from nature, where under mild conditions photosynthetic organisms (e.g., algae) use the energy of the sun to produce complex chemicals out of simple building blocks: atmospheric carbon dioxide or nitrogen and water. Rather than using electricity from solar cells to enable the electrochemical production of hydrogen and carbon compounds, these technologies combine everything necessary in an integrated conversion system to go directly from sunlight to the final chemical product of choice. This offers a genuine route to minimize losses over value chains, but requires possibly a few decades to reach a technology readiness level of 9. Recent developments in directed evolution of enzymes have shown tremendous progress, which has been made in biochemical routes to produce chemicals and fuels directly from carbon dioxide, water, and nitrogen.
Therefore, on short notice power-2-heat (P2H) will be the first step in the electrification of the chemical industry. P2H simply defines a process whereby generated power is used for heating and cooling applications. Classically, people would think of heat pumps or boilers but in the chemical industry higher temperatures are needed. For example, for the production of ethylene in a process called steam cracking temperatures as high as 900°C are required with a power input of several 100 MW.11 This implies different technologies, different concepts, and different materials compared to power-2-X (P2X) concepts,12 where electricity is used directly. Hydrogen is the best example where numerous P2H and P2X options have been advocated, as schematically illustrated in Figure 3. With the current economics and electricity prices, electrolysis makes not yet much sense as it is a factor three more expensive than producing hydrogen from steam reforming. Again there is more than money that is driving the World, but it is an aspect that should not be hidden as it is often ignored in discussions.
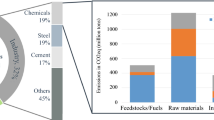
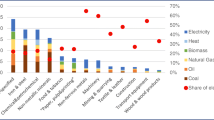
Figure 3 points also to another difficulty, which is not related to economics as: What is in the end the most sustainable solution for a given chemical, albeit hydrogen, olefins, or anything else? This means that the GHG emissions of different options need to be compared fairly to answer one of the key questions for the coming years: “How can these emissions be reduced on short notice without seriously affecting the entire economy, including the supply of consumer goods and services?” Essential in this context is the greenhouse gas (GHG) protocol, a global standard for companies and organizations to measure and manage their GHG emissions and become more efficient, resilient, and prosperous, schematically illustrated in Figure 4. In short, the GHG protocol is a bookkeeping system that divides the emissions in three categories: Scope 1, Scope 2, and Scope 3. Simplified for most chemical processes, Scope 1 is related to the process itself, Scope 2 is related to the electricity used, Scope 3 is related to the feedstock production and transport. To make that a bit more tangible with hydrogen as example electrolysis with renewable electricity would result in zero Scope 2 emissions, while if gray electricity would be used the Scope 2 emissions would become enormous. If a coal-based power plant would be used, the emissions of electrolyser would be substantially larger than from steam methane reforming. The Scope 3 emissions also point to an important aspect that is related to the emissions related to the used feedstock. For example, if a source such as plastic waste is used instead of a fossil starting material, Scope 3 emissions will be very different. In processes such as steam cracking, which is the dominant process for the production of ethylene, propylene, 1,3-butadiene, and aromatics, the Scope 3 emissions are higher than the sum of Scope 1 and 2 emissions if a fossil feedstock is used. Note that this GHG protocol will also be used to make decisions related to which materials that will be used in the processes itself. Also for their production Scope 1, Scope 2, and Scope 3 emissions will be key elements to make decisions which option is favored.
Materials for power-2-heat
From a materials point of view, research has pointed out that material scarcity could be a limiting factor to the expansion of renewable energy systems13 also for P2H concepts that can be a major show stopper, or at the very least be a delaying factor. As stated previously, P2H compromises the upgrading of waste heat streams, heat and cold storage, and the direct transformation of electricity to heat (e.g., induction or restive heating). In the past lustrum, the two chemicals that attracted most attention in this context are ethylene and hydrogen. Therefore, it is best to focus on those two case studies as reference for others.
Materials for P2H of hydrogen production
For hydrogen, a distinction must be made between production processes that require pure hydrogen, such as ammonia, or processes that build on syngas, such as methanol. In the case syngas is the objective, Haldor Topsoe and also researchers from DTU electrified steam methane reforming (SMR) using an FeCrAl tube that provides a compact platform for the production of greener syngas and all syngas-derived chemicals.14 If renewable electricity is used, an electrified SMR process based on natural gas could reduce CO2 emissions per kg H2, depending on the efficiency and fuel15 used in the classical SMR process. This is not the only solution that has been proposed, as demonstrated by Vinum et al., induction heating of ferromagnetic catalyst particles enables heat delivered directly to the catalytic site.16 However, scalability is also essential for the overall energy efficiency, as shown by Almind et al.17 and induction heating has certain limitations. Meloni et al. demonstrated similar inversion of the thermal gradient using microwaves to heat a structured Ni/SiC catalyst.18 This was also demonstrated by Renda et al., heating a catalyzed SiC element by an ohmic resistance.19 A highly robust system was demonstrated by Surov et al. utilizing a high-temperature plasma torch to drive a reforming reaction without a catalyst.20
This short overview of different P2H concepts illustrates that from a material point of view no drastic changes of today’s situation would be needed, which is obviously a big plus. The latter partly explains why P2H will be the first step in the electrification of the chemical industry. Other advantages come from process intensification. For example, the integrated ohmic heating seems to enable substantial reductions in reactor volume by up to two orders of magnitude compared to equivalent fired reformer.14
For producing pure hydrogen, P2H concepts seem to be less attractive from a GHG emission point of view unless one considers the production of blue hydrogen in which the CO2 is captured and stored (CCS). Economically this makes today a lot more sense than electrolysis, even with costs for capturing and storing the CO2 of 100€ per ton. As this allows to keep on using the current reactor materials and only slightly modified heterogenous catalysts, this is a path that is seriously considered by several companies. However, the Scope 3 emissions related to the production of natural gas will always be substantial. To reduce Scope 3 emissions, the use of biogas is of interest because of the large volumes that are available and the fact that biogas production for heating or electricity production is oversubsidized and governments are thus looking for higher value applications. Biogas, a mixture of mainly CH4 (40 to 75 vol%) and CO2 (25 to 60 vol%) typically produced via anaerobic digestion of organic waste material, is envisioned to be one of the key resources in achieving (e.g., the European Union’s 2030 decarbonization and renewable energy targets). Biogas is nowadays typically combusted to locally produce electricity and/or heat but could also be (more effectively) valorized through super dry reforming (SDR) or dry reforming (DR).21 Key challenges include developing stable and selective catalyst materials able to deal with varying biogas compositions, and the operations under fully dry conditions.
One reflection that needs to be made about hydrogen is that today a hydrogen economy is not embraced by all actors. Indeed, it is simply not wise today because besides the production, also the current storage approaches are energy-intensive. Gas storage requires hydrogen compression up to 350–700 bars, amounting to the energy usage of 2–4 kWh/kg H2. Hydrogen liquefaction, on the other hand, occurs at − 253°C (1 atm) and requires a complex technical plant that uses up to 12 kWh/kg H2.
Materials for P2H of ethylene production
The dominant production process for ethylene is currently steam cracking, which is also the dominant production process for many other base chemicals, such as propylene, C4s (e.g., butenes), and aromatics (i.e., xylenes). Electrification of steam cracking has received enormous attention with recent press releases of almost all the major chemical players that they are working on different aspects and options (e.g., BASF, Sabic, Shell, Dow, and total). It is clear that the potential emission reduction by stepwise improvements to the conventional process is limited unless CCS is used.22 For example, by combining high emissivity coatings with novel aluminum-based alloys, reactive coatings in the coil,23 3D machined reactors, and novel process design, at best can result in 30% emission reductions as shown by Brown et al.24 So, more drastic innovations are needed. Electrification might offer the opportunity to drastically change the process to enhance energy efficiency and product yield. An alternative is the indirect use of electricity via renewable hydrogen. Even though requiring adaptations to the current technology (i.e., hydrogen combustion is fundamentally different from conventional fuels, such as methane), it would lead to significant reductions in the GHG emissions. The product yield, on the other hand, will hardly be improved as this is limited by the allowable furnace tube skin temperature. This also applies to conductive or inductive heating of the furnace tubes. This makes the roto dynamic reactor (RDR) developed by Coolbrook a lot more interesting.25 Although they can be made from the same materials as cracking coils, an ethylene yield increase of up to 4 wt% compared to the best available technology seems possible. This rotor–stator–diffuser works as a bad compressor in which the electrically energy is via the rotor transformed into heat in combination with shock waves. This revolutionary approach of energy transfer via the dynamic action of rotating blades could be of interest for catalytic processes as well. Using a catalytic process would be another way to reduce the energy consumption of olefin production. This may also offer many other unexpected benefits appropriately linked with the nature of energy–catalyst interactions. However, implementing an unconventional energy supply to drive a reaction brings many challenges for catalyst design and upscaling. Traditionally, the development of heterogeneous catalysts has been considered part art, part science, and mostly experience. In the future, we need to move from “data and experience for decision” to “model for decision and experimental design.” The recent progress in the analytical tools (including the inline local temperature measurements), new enabling technologies (including high-throughput experimentation and 3D printing), and access to higher computational power bring many opportunities to achieve the goals via machine learning to design new generations of better performing and stable catalyst materials.
In any case, if olefin production would be electrified or a catalytic alternative would be used the process needs to run 8000 h per year. This means also during periods when green electricity is very expensive or not available. Battery energy storage systems have been investigated as storage solutions due to their responsiveness, efficiency, and scalability.26 Potentially cheap batteries are iron–air batteries composed of cells filled with thousands of iron pellets that are exposed to air and create rust. The oxygen is then removed, reverting the rust to iron. Controlling this process allows the battery to be charged and discharged. Recently, an MIT start-up Form Energy Inc. says they will be capable of solving one of the most elusive problems facing renewable energy: cheaply storing large amounts of electricity to power grids when the sun is not shining and wind is not blowing. For a lithium-ion battery cell, the workhorse of electric vehicles, and today’s grid-scale batteries, the nickel, cobalt, lithium, and manganese minerals used currently cost between $USD50 and $USD80 per kilowatt-hour of storage, according to analysts. Using iron, Form Energy Inc. believes it will spend less than $6 per kilowatt-hour of storage on materials for each cell. Packaging the cells together into a full battery system will raise the price to less than $20 per kilowatt-hour, a level at which academics have said renewables plus storage could fully replace traditional fossil-fuel-burning power plants. The future will tell if this indeed will lead to the expected breakthrough.
Materials for power-2-X
Materials for P2X of hydrogen production
Water splitting has gained a lot of attention in recent years as it provides a viable way to produce H2 and O2 from H2O with renewable electricity.27,28,29 Once this renewable hydrogen is produced at the required scale existing or very similar chemical processes can be conducted. Examples include methanol, methane, and ammonia synthesis. However, H2O electrolysis has to be further improved to be fully deployed at the large scale and to be fully competitive with current production processes based on fossil resources. One of the main challenges is to lower the overpotential, which results in the unnecessary loss of renewable electricity for both the hydrogen evolution reaction (HER) and oxygen evolution reaction (OER). Catalysts based on Pt (HER), Ir (OER), and Ru (OER) are the most used, but are difficult to implement in large-scale applications due to their high cost and related scarcity. Hence, materials scientists are searching to replace these expensive (noble) elements with earth-abundant first row transition metal oxides/hydroxides and/or mixed oxides/hydroxides30,31,32,33 Based on the literature, one of the best performing HER electrocatalysts is Ni–Mo, which approaches the activity of Pt-based catalysts.34,35,36 While for OER, Ni–Fe, Ni–S, and Ni–Fe–S have been found to be good electrocatalysts, these materials still get trumped by Ir- and Ru-based electrocatalysts.37,38,39 Clearly, more research has to be done to find alternative and cheap electrocatalyst materials to be implemented in future e-refinery plants and research programs have to be developed in which materials research goes hand in hand with process upscaling as well as long-term durability tests under dynamic conditions (i.e., day versus night and summer versus winter variations).
Hence, electrode stability is a very important aspect for materials research, and electrocatalyst degradation has to be fully understood before such alternative systems could be commercially deployed. Recent characterization studies have found that catalyst components may either be oxidized or leached into the electrolyte solution, and that electrocatalysts turn from their precursor state into their active state during the HER and OER reaction. For example, the dynamic nature of electrode catalysts was recently shown for the OER reaction, where Fe dissolution and redeposition on a hydr(oxy)oxide cluster could be demonstrated.40 In situ spectroscopy has proven to be very useful to study electrocatalysts at work, as has been summarized in a recent review of Zhu et al. for Ni–Fe-based OER catalysts.41 For example, Hollmann et al. used In situ Raman spectroscopy to identify an NiO(OH) species in the most active OER catalyst,42 while Rao et al. using surface-enhanced infrared absorption (SEIRA) spectroscopy identified an O–O intermediate on a coordinated unsaturated Ru in an OER catalyst.43 Huang et al. revealed with shell-isolated nanoparticle-enhanced Raman spectroscopy (SHINERS) the presence of (su)peroxide species on Pt during electrochemical oxidation.44 Finally, the Domke group used tip-enhanced Raman spectroscopy (TERS) to study the behavior of Au under H2O electrolysis conditions.45 TERS revealed the nanoscale AuOx (e.g., Au2O3 and Au2O) formation and the reversible transformation toward Au metal when applying a reduction potential. Recently, Wijten et al. have acquired new insights in the effect of electrode potential, electrolyte type, and pH on MoO42− leaching in Ni–Mo HER catalysts.46,47,48 This was possible, as illustrated in Figure 5, by using, a.o., In situ atomic force microscopy (AFM), which was coupled to solution UV–vis spectroscopy.
Characterization of the stability of electrode materials for P2X of H2 and ethylene production: (a) In situ atomic force microscopy (AFM) of the hydrogen evolution reaction (HER) during H2O electrolysis over a Ni–Mo/Ti electrocatalyst in KOH electrolyte with increasing molarity, showing an increasing surface roughening, accompanied with Mo leaching, with time and47 (b) In situ surface-enhanced Raman spectroscopy (SERS) of the CO2 electroreduction (CO2RR) process over a Cu electrocatalyst, showing the influence of the electrode potential on the surface species as well as the yield toward ethylene.57
Materials for P2X of ethylene production
Electrochemical CO2 reduction reaction (CO2RR) also holds great promise for sustainable energy conversion and storage.49,50,51,52 If this process could be realized at a reasonable cost and efficiency, e-chemicals and e-fuels could be made in a sustainable way, thus allowing for a closed-loop cycle for carbon, as shown in Figure 1.53 In particular, the production of C2 products, such as ethylene and ethanol, would be attractive for applications in energy storage and conversion as well as for the manufacturing of chemicals,54 although C1 products, such as formate, would also open many possibilities. The question is which chemical building blocks can be made in an effective manner, according to the e-Chemistree of Figure 2, to make sure that we can make the wide variety of chemicals, fuels, and materials needed for our future-renewable society.
The CO2RR process is studied in great detail but still presents major challenges as (1) current catalysts require large overpotentials; (2) the selectivity toward C2 products is low; and (3) HER is a major competing process. So far, copper-based CO2RR catalysts are the only materials that show significant selectivity toward, e.g., C2 products but major challenges still exist. These include mechanistic insights into the activity and selectivity toward C2+ products. Many studies aim to shed light on the possible reaction pathways through In situ spectroscopy and microscopy.55,56 For example, IR spectroscopy revealed that adsorbed CO are key intermediates. Furthermore, a OCCOH* signal on Cu(100) surfaces has been related to CO dimerization, a necessary step for making C2 products, while glyoxal and acetaldehyde are also proposed as reaction intermediates. Recently, An et al.57 have used In situ surface-enhanced Raman spectroscopy (SERS) and AFM to investigate the rapid changes taking place at a Cu electrode surface during CO2RR. SERS revealed that a highly dynamic CO intermediate is related to C=C coupling and ethylene production at high cathodic bias, whereas lower cathodic bias resulted in gaseous CO production from isolated and static CO surface species. This electrocatalyst dynamics is illustrated in Figure 5.
Important to note is that electrocatalytic processes are dominated by the catalyst–electrolyte interface chemistry, which includes charge transfer and molecular conversion processes.58These processes, including the mechanistic pathways, are also pH-dependent, as has been recently discussed in the work of Chan et al.59 Estimations of the pH near the electrode surface have been made based on linear extrapolation of the pH drop beyond the diffusion layer, which indicate that the pH lowers during reaction.60 However, an accurate measurement of the local pH variations at the electrode surface is still lacking. Such measurements are crucial for the design of new or improved electrocatalyst materials that can access novel reaction pathways, thereby steering the product slate toward the wanted C1 or C2 products, by exploiting the effect of pH on the stability of the reaction intermediates.
Concluding remarks
We are on the verge of a new era for the chemical industry. In the coming decade, an industrial revolution will unfold, driven by defossilization, sustainability, and circularity. The transformation of the chemical industry is not without risk, as manufacturing processes that have been developed over more than a century must be replaced by methods that currently only have been proven on lab scale or are simply not existing today. It should be stressed that safety should remain the number one priority of the chemical industry and that new production facilities should also obey this general principle. Therefore, researchers all over the world need to develop new techniques of which only a few are likely to become the new best available technologies. This high-risk, high-gain, and large-scale effort can only succeed with an appropriate (global) policy support that stimulates the scientific and technological developments and distributes the related risks. The huge financial investments will be next to material scarcity and long depreciation times of investments the potentially delaying factors to really go quickly to the e-chemistree, as proposed in this work. Moreover, the increasing need for green electricity is a major challenge to reduce GHG emissions of chemical processes. For P2H concepts, the same materials that are currently in place will be needed. However, energy buffering and storage will be essential to make sure that the variable renewable energy does not require chemical plants need to stop, but let us run them safe and use their capital investment in optimal way. Cheap solutions such as those involving iron–air batteries that are capable of affordable, long-duration power storage would be a game changer and similar discoveries are expected to take place in the decades to come. For P2X concepts, totally new electrode materials and reactor concepts have to be designed. Electrode materials are preferentially composed of earth-abundant elements, and which are not only very selective toward the formation of key C1 and C2 intermediates, such as CO, formate, and ethylene, but also very stable. This requires both theoretical and experimental efforts, as well as the training of a whole new generation of materials scientists, skilled in electrosynthesis.
References
IEA: World Energy Outlook 2020, in World Energy Outlook (International Energy Agency, Paris, 2020)
IEA: Renewables 2020, in Renewables (International Energy Agency, Paris, 2020), p. 172
E. Parliament: Fit for 55, (2021)
K.M. Van Geem, V.V. Galvita, G.B. Marin, Making chemicals with electricity. Science 364, 734 (2019)
M. Bonheure, L.A. Vandewalle, G.B. Marin, K.M. Van Geem, Dream or reality? Electrification of the chemical process industries. Chem. Eng. Prog. 117, 37 (2021)
Z.J. Schiffer, K. Manthiram, Electrification and decarbonization of the chemical industry. Joule 1, 10 (2017)
A.D. Pee, D. Pinner, O. Roelofsen, K. Somers, E. Speelman, M. Witteveen, Decarbonization of Industrial Sectors: The Next Frontier (McKinsey & Company, Amsterdam, 2018), p. 68
H.-J. Arpe, Industrial Organic Chemistry, 5th ed. (2010)
H.C. Erythropel, J.B. Zimmerman, T.M. de Winter, L. Petitjean, F. Melnikov, C.H. Lam, A.W. Lounsbury, K.E. Mellor, N.Z. Janković, Q. Tu, L.N. Pincus, M.M. Falinski, W. Shi, P. Coish, D.L. Plata, P.T. Anastas, The Green ChemisTREE: 20 years after taking root with the 12 principles. Green Chem. 20, 1929 (2018)
R.F. Service, Artificial leaf turns sunlight into a cheap energy source. Science 332, 25 (2011)
I. Amghizar, L.A. Vandewalle, K.M. Van Geem, G.B. Marin, New trends in Olefin production. Engineering 3, 171 (2017)
TNO: Empowering The Chemical Industry: Opportunities for Electrification, ed. by K.V. Kranenburg, E. Schols, H. Gelevert, R.D. Kler, Y.V. Delft, M. Weeda (TNO, Amsterdam, 2016), p. 32
A. Valero, A. Valero, G. Calvo, A. Ortego, Material bottlenecks in the future development of green technologies. Renew. Sustain. Energy Rev. 93, 178 (2018)
S.T. Wismann, J.S. Engbæk, S.B. Vendelbo, F.B. Bendixen, W.L. Eriksen, K. Aasberg-Petersen, C. Frandsen, I. Chorkendorff, P.M. Mortensen, Electrified methane reforming: a compact approach to greener industrial hydrogen production. Science 364, 756 (2019)
M.M.K. Spath P.L., Life Cycle Assessment of Hydrogen Production via Natural Gas Steam Reforming (2001)
M.G. Vinum, M.R. Almind, J.S. Engbæk, S.B. Vendelbo, M.F. Hansen, C. Frandsen, J. Bendix, P.M. Mortensen, Dual-function cobalt-nickel nanoparticles tailored for high-temperature induction-heated steam methane reforming. Angew. Chem. Int. Ed. 57, 10569 (2018)
M.R. Almind, S.B. Vendelbo, M.F. Hansen, M.G. Vinum, C. Frandsen, P.M. Mortensen, J.S. Engbæk, Improving performance of induction-heated steam methane reforming. Catal. Today 342, 13 (2020)
E. Meloni, M. Martino, A. Ricca, V. Palma, Ultracompact methane steam reforming reactor based on microwaves susceptible structured catalysts for distributed hydrogen production. Int. J. Hydrogen Energy 46, 13729 (2021)
S. Renda, M. Cortese, G. Iervolino, M. Martino, E. Meloni, V. Palma, Electrically driven SiC-based structured catalysts for intensified reforming processes. Catal. Today (2020)
A.V. Surov, S.D. Popov, V.E. Popov, D.I. Subbotin, E.O. Serba, V.A. Spodobin, G.V. Nakonechny, A.V. Pavlov, Multi-gas AC plasma torches for gasification of organic substances. Fuel 203, 1007 (2017)
K. Verbeeck, L.C. Buelens, V.V. Galvita, G.B. Marin, K.M. Van Geem, K. Rabaey, Upgrading the value of anaerobic digestion via chemical production from grid injected biomethane. Energy Environ. Sci. 11, 1788 (2018)
I. Amghizar, J. Dedeyne, D.J. Brown, G.B. Marin, K.M. Van Geem, Sustainable innovations in steam cracking: CO2 neutral olefin production. React. Chem. Eng. 5, 239 (2020)
C.M. Schietekat, S.A. Sarris, P.A. Reyniers, L.B. Kool, W.Q. Peng, P. Lucas, K.M. Van Geem, G.B. Marin, Catalytic coating for reduced coke formation in steam cracking reactors. Ind. Eng. Chem. Res. 54, 9525 (2015)
D.J. Brown, I. Amghizar, G.B. Marin, J. Dewulf and K. Van Geem, How to Reduce CO2 Emissions of Steam Cracking Furnaces: The Million Dollar Question, in AIChE Spring Meeting (2021)
J. Seppala, J. Hiltunen, V.-M. Purola: Process and rotary machine type reactor, (Coolbrook Oy United States, 2016), p. 1
B. Faessler, Stationary, second use battery energy storage systems and their applications: a research review. Energies 14, 2335 (2021)
L.A. King, M.A. Hubert, C. Capuano, J. Manco, N. Danilovic, E. Valle, T.R. Hellstern, K. Ayers, T.F. Jaramillo, A non-precious metal hydrogen catalyst in a commercial polymer electrolyte membrane electrolyser. Nat. Nanotechnol. 14, 1071 (2019)
B. You, Y. Sun, Innovative strategies for electrocatalytic water splitting. Acc. Chem. Res. 51, 1571 (2018)
J. Kibsgaard, I. Chorkendorff, Considerations for the scaling-up of water splitting catalysts. Nat. Energy 4, 430 (2019)
X. Zou, Y. Zhang, Noble metal-free hydrogen evolution catalysts for water splitting. Chem. Soc. Rev. 44, 5148 (2015)
L. Han, S. Dong, E. Wang, Transition-metal (Co, Ni, and Fe)-based electrocatalysts for the water oxidation reaction. Adv. Mater. 28, 9266 (2016)
B.M. Hunter, H.B. Gray, A.M. Müller, Earth-abundant heterogeneous water oxidation catalysts. Chem. Rev. 116, 14120 (2016)
N.-T. Suen, S.-F. Hung, Q. Quan, N. Zhang, Y.-J. Xu, H.M. Chen, Electrocatalysis for the oxygen evolution reaction: recent development and future perspectives. Chem. Soc. Rev. 46, 337 (2017)
A.R. Zeradjanin, J.-P. Grote, G. Polymeros, K.J.J. Mayrhofer, A critical review on hydrogen evolution electrocatalysis: re-exploring the volcano-relationship. Electroanalysis 28, 2256 (2016)
C.C.L. McCrory, S. Jung, I.M. Ferrer, S.M. Chatman, J.C. Peters, T.F. Jaramillo, Benchmarking hydrogen evolving reaction and oxygen evolving reaction electrocatalysts for solar water splitting devices. J. Am. Chem. Soc. 137, 4347 (2015)
M. Zeng, Y. Li, Recent advances in heterogeneous electrocatalysts for the hydrogen evolution reaction. J. Mater. Chem. A 3, 14942 (2015)
W. Zhou, X.-J. Wu, X. Cao, X. Huang, C. Tan, J. Tian, H. Liu, J. Wang, H. Zhang, Ni3S2 nanorods/Ni foam composite electrode with low overpotential for electrocatalytic oxygen evolution. Energy Environ. Sci. 6, 2921 (2013)
M.W. Kanan, D.G. Nocera, In situ formation of an oxygen-evolving catalyst in neutral water containing phosphate and Co2+. Science 321, 1072 (2008)
D.R. Kauffman, D. Alfonso, D.N. Tafen, J. Lekse, C. Wang, X. Deng, J. Lee, H. Jang, J.-S. Lee, S. Kumar, C. Matranga, Electrocatalytic oxygen evolution with an atomically precise nickel catalyst. ACS Catal. 6, 1225 (2016)
D.Y. Chung, P.P. Lopes, P. Farinazzo Bergamo Dias Martins, H. He, T. Kawaguchi, P. Zapol, H. You, D. Tripkovic, D. Strmcnik, Y. Zhu, S. Seifert, S. Lee, V.R. Stamenkovic, N.M. Markovic, Dynamic stability of active sites in hydr(oxy)oxides for the oxygen evolution reaction. Nat. Energy 5, 222 (2020)
K. Zhu, X. Zhu, W. Yang, Application of In situ techniques for the characterization of NiFe-based Oxygen Evolution Reaction (OER) electrocatalysts. Angew. Chem. Int. Ed. 58, 1252 (2019)
D. Hollmann, N. Rockstroh, K. Grabow, U. Bentrup, J. Rabeah, M. Polyakov, A.-E. Surkus, W. Schuhmann, S. Hoch, A. Brückner, From the precursor to the active state: monitoring metamorphosis of electrocatalysts during water oxidation by In situ spectroscopy. ChemElectroChem 4, 2117 (2017)
R.R. Rao, M.J. Kolb, L. Giordano, A.F. Pedersen, Y. Katayama, J. Hwang, A. Mehta, H. You, J.R. Lunger, H. Zhou, N.B. Halck, T. Vegge, I. Chorkendorff, I.E.L. Stephens, Y. Shao-Horn, Operando identification of site-dependent water oxidation activity on ruthenium dioxide single-crystal surfaces. Nat. Catal. 3, 516 (2020)
Y.-F. Huang, P.J. Kooyman, M.T.M. Koper, Intermediate stages of electrochemical oxidation of single-crystalline platinum revealed by In situ Raman spectroscopy. Nat. Commun. 7, 12440 (2016)
J.H.K. Pfisterer, M. Baghernejad, G. Giuzio, K.F. Domke, Reactivity mapping of nanoscale defect chemistry under electrochemical reaction conditions. Nat. Commun. 10, 5702 (2019)
J.H.J. Wijten, R.L. Riemersma, J. Gauthier, L.D.B. Mandemaker, M.W.G.M.T. Verhoeven, J.P. Hofmann, K. Chan, B.M. Weckhuysen, Electrolyte effects on the stability of Ni-Mo cathodes for the hydrogen evolution reaction. Chemsuschem 12, 3491 (2019)
J.H.J. Wijten, L.D.B. Mandemaker, T.C. van Eeden, J.E. Dubbeld, B.M. Weckhuysen, In situ study on Ni-Mo stability in a water-splitting device: effect of catalyst substrate and electric potential. Chemsuschem 13, 3172 (2020)
J.H.J. Wijten, I. Garcia-Torregrosa, E.A. Dijkman, B.M. Weckhuysen, Basicity and electrolyte composition dependent stability of Ni-Fe-S and Ni-Mo electrodes during water splitting. ChemPhysChem 21, 518 (2020)
D.T. Whipple, P.J.A. Kenis, Prospects of CO2 utilization via direct heterogeneous electrochemical reduction. J. Phys. Chem. Lett. 1, 3451 (2010)
E.V. Kondratenko, G. Mul, J. Baltrusaitis, G.O. Larrazábal, J. Pérez-Ramírez, Status and perspectives of CO2 conversion into fuels and chemicals by catalytic, photocatalytic and electrocatalytic processes. Energy Environ. Sci. 6, 3112 (2013)
R.M. Arán-Ais, D. Gao, B. Roldan Cuenya, Structure- and electrolyte-sensitivity in CO2 electroreduction. Acc. Chem. Res. 51, 2906 (2018)
C. Xie, Z. Niu, D. Kim, M. Li, P. Yang, Surface and interface control in nanoparticle catalysis. Chem. Rev. 120, 1184 (2020)
A. Ramirez, S.M. Sarathy, J. Gascon, CO2 derived E-fuels: research trends, misconceptions, and future directions. Trends Chem. 2, 785 (2020)
Y. Hori, in Electrochemical CO2 Reduction on Metal Electrodes, in Modern Aspects of Electrochemistry, ed. by C.G. Vayenas, R.E. White, M.E. Gamboa-Aldeco (Springer, New York, 2008), p. 89.
Y.W. Choi, H. Mistry, B. Roldan Cuenya, New insights into working nanostructured electrocatalysts through operando spectroscopy and microscopy. Curr. Opin. Electrochem. 1, 95 (2017)
B. Roldan Cuenya, F. Behafarid, Nanocatalysis: size- and shape-dependent chemisorption and catalytic reactivity. Surf. Sci. Rep. 70, 135 (2015)
H.Y. An, L.F. Wu, L.D.B. Mandemaker, S. Yang, J. de Ruiter, J.H.J. Wijten, J.C.L. Janssens, T. Hartman, W. van der Stam, B.M. Weckhuysen, Sub-second time-resolved surface-enhanced raman spectroscopy reveals dynamic CO intermediates during electrochemical CO2 reduction on copper. Angew. Chem. Int. Ed. 60, 16576 (2021)
Y.J. Sa, C.W. Lee, S.Y. Lee, J. Na, U. Lee, Y.J. Hwang, Catalyst–electrolyte interface chemistry for electrochemical CO2 reduction. Chem. Soc. Rev. 49, 6632 (2020)
H. Peng, M.T. Tang, X. Liu, P. Schlexer Lamoureux, M. Bajdich, F. Abild-Pedersen, The role of atomic carbon in directing electrochemical CO(2) reduction to multicarbon products. Energy Environ. Sci. 14, 473 (2021)
H. Ooka, M.C. Figueiredo, M.T.M. Koper, Competition between hydrogen evolution and carbon dioxide reduction on copper electrodes in mildly acidic media. Langmuir 33, 9307 (2017)
Acknowledgments
The research leading to these results has received funding from the European Research Council under the European Union’s Horizon 2020 research and innovation programme/ERC Grant Agreements No. 818607 “ERC OPTIMA” and No. 820444 “Energy-X” (CSA), as well as from the Advanced Research Center Chemical Building Blocks Consortium (ARC CBBC) as well as The Netherlands Center for Multiscale Catalytic Energy Conversion (MCEC), which are both (co-) funded by The Netherlands Organisation for Scientific Research (NWO).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no financial interests.
Rights and permissions
Open access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Van Geem, K.M., Weckhuysen, B.M. Toward an e-chemistree: Materials for electrification of the chemical industry. MRS Bulletin 46, 1187–1196 (2021). https://doi.org/10.1557/s43577-021-00247-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/s43577-021-00247-5