Abstract
There is major fire safety concern about failure propagation of thermal runaway in multicell lithium-ion batteries. This article overviews the passive fire-protection approach based on thermal insulation by intumescent coating materials and fire blankets for viable failure resistance. The intumescent coating will expand (up to 100× on heating) to form a thick, porous char layer and act as a thermal barrier to insulate the substrate. It is also used to seal around openings in a wall or floor to impede the spread of fire and smoke. High-temperature fire blankets are made of noncombustible or flame-resistant fabric materials (e.g., aramids, fiberglass, amorphous silica, preoxidized carbon, and mineral fibers). Both working intumescent coating and fire blankets can block a significant portion (typically 60 to 90%) of the incident heat. Impact-resistant high-strength fabrics, in either soft or rigid forms, can also be used as parts of multilayer protection assembly. Thus, multilayer assemblies can be used for various commercial products, including passive and active fire-protection blankets, battery-cell partitions, confinement bags and containers, packaging materials, and personal protective equipment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Despite their numerous advantages, lithium (Li)-ion batteries are subject to a catastrophic failure mode (i.e., thermal runaway), which may cause fire and explosion under certain conditions.1,2,3,4,5,6,7 A risk assessment1 of the Li-ion batteries used in electric vehicles (EVs) focused on thermal runaway, toxic gas emission, fire propagation, and explosion, among others, and suggested possible mitigation or protection strategies. Thermal runaway8,9,10,11,12,13,14,15 is a series of rapid self-heating (exothermic) processes, within the Li-ion cell itself that can be caused by an internal defect or intentional or unintentional abuse, including but not limited to, electrical overcharge, overheating, internal short circuit, and mechanical damage. A number of cells are packed together in various configurations (parallel and/or series connected) to form a module.16 The initial cell failure may increase the temperature of adjacent cells, thus, causing a cell-to-cell thermal runaway propagation,17,18,19,20,21,22 which results in a much more serious damage.
As the temperature of the Li-ion cell increases and, in turn, the inner pressure increases, the safety valve, if provided, opens at a certain threshold pressure, or the casing of the cell swells and ruptures to relieve the pressure.1,2,23,24 As a result, strong venting of the electrolyte containing flammable organic solvents occurs in the forms of aerosol droplets (smoke) and vapor, accompanied by gases, including H2, CO, and CO2, and decomposed hydrocarbons. If the rupture of the cell case occurs explosively, ballistic projectiles hurl into surroundings. Therefore, for the Li-ion batteries undergoing a thermal runaway, there are all three elements of the fire triangle: fuel, oxidizer, and heat, which are the conditions necessary for fire to occur. If the vaporized electrolyte and flammable gases (fuels) mix with oxygen (oxidizer) from the ambient air with the fuel–air ratio within the fuel-lean and rich flammability limits, the electric spark or hot surface (heat) can ignite the mixture, thus causing a fire. If the fuel–air mixture is accumulated in a confined or semi-confined space and ignited at a delayed time, a gas explosion can occur, resulting in severe damage.24
Many research papers and reviews on possible mitigation or protection of thermal runaway and cell-to-cell propagation have been published in the literature. There are two distinctively different approaches: cooling and thermal isolation (insulation). Most of the mitigation methods of thermal runaway (and its propagation) through passive thermal management system use various cooling media, including air, liquid, and phase-change materials.16,25,26,27,28,29,30 For example, a vaporizing heatsink product, consisted of a polymer shell containing carbon fiber wicks and liquid installed between rows of Li-ion cells, has been developed for passive resistance to thermal runaway propagation in Li-ion batteries.
Heat dissipation methods can regulate the temperature of the battery packs and prevent the thermal runaway propagation. Aluminum plates were assembled into battery modules as heatsinks, and the effect of plate thickness on thermal runaway mitigation was investigated experimentally and numerically.31,32,33,34 It was concluded that thermal runaway propagation in a battery module can be prevented by proper design of passive thermal management system.
Cylindrical battery cells, wrapped in a thermally and electrically insulating mica sleeve and fixed in conductive aluminum heatsink, reduced the temperature of the adjacent cells during thermal runaway.32 The insulating material reduced the heat transfer from the failed cell and the massive heatsink dissipated the heat, thus, reducing the effects on the neighboring cells. Although large thermal conductivity and heat capacity of the metal enabled enhanced heat transfer and storage, its large density made the system heavy.
Experiments on the designed battery modules showed34 that adding the insulation material of aerogel postponed the thermal runaway propagation, but there was no obvious delay by adding the liquid cooling plate only, and their combination prevented the thermal runaway propagation. This approach also used both cooling and insulation effects.
The second approach to the mitigation (resistance) of the thermal runaway propagation by thermal isolation (insulation) of the failed cell has not yet been studied well. There are various heat-blocking methods and materials, which can be assembled as a multilayer thermal barrier, to reduce the heat transfer to prevent or resist the thermal runaway propagation. This article focuses on intumescent coating materials and high-temperature fire blanket fabric materials, including ballistic textiles, intended to be used for Li-ion battery cells, modules, packs, and their packaging. According to the nature of the article, no datasets were generated or analyzed for Li-ion battery applications during the current study. Nonetheless, the previous works on both intumescent coating and fire blanket materials are based on the heat-blocking mechanism, i.e., an element of the fire triangle. Thus, these conventional methods are well suited to the special case of Li-ion batteries in thermal runaway propagation, where the heat is generated within the cells and conducted to the adjacent cells as well as the heat from external fire, if any. The goal of this article is to provide a summary of previous results to stimulate activities in the related research.
Intumescent coating materials
Under the action of heat, intumescent coatings35,36,37,38,39,40 will expand and form a thick, porous char layer with a very low thermal conductivity, thus, acting as a thermal barrier to insulate the substrate. Intumescent coatings can swell up to 100 times on heating (e.g., from 1-mm-thick to 10-cm-thick foam). The substrate will be protected against high temperature rise and exposure to oxygen within a certain period. Applying the intumescent coating is an efficient way of providing fire retardancy to flammable materials. Therefore, they have a wide application for protecting metals, plastics, textiles, and wood against fire.
The outlines of the intumescent chemistry were described in the classical Vandersall review.35,36,37,38,39,40 In the general and simplified summary, (1) the acid source first decomposes and forms mineral acid after being exposed to incident heat, (2) the generated acid dehydrates the carbon source to form esters, (3) the ester decomposes to form carbon and releases the acid, (4) the resinous material melts to form a film of skin over the carbonaceous material, and (5) the gas-generating blowing agents release inert gases, which will puff the coating and form a foam structure char layer.
In a previous study,41,42 intumescent coating’s thermal insulation performance, expansion rate, and mass loss rate have been measured using a laboratory-scale heating apparatus, consisting of a radiant cone heater and a sample holder (Figure 1a), which is attached to a precision weighing balance. Six K-type thermocouples, inserted from the bottom surface of the sample to different heights, measure the in-depth temperatures of the char layer, and a water-cooled 3.18-mm-diameter heat-flux transducer determines the heat transmitted through the char (Figure 1b).
(a) Experimental setup for intumescent coating materials. The cone heater and a laser-based coating-surface detection system rises in synch with the swelling intumescent coating to maintain the heater-to-sample distance and, in turn, a constant incident radiant flux. Reprinted with permission from Reference 41. © 2018 SAGE Publications. (b) Horizontal (top view) and vertical (side view) locations of the heat-flux transducer and six thermocouples.
The thermal insulating performance of the expanded char layer of intumescent coatings can be evaluated by the heat-blocking efficiency, HBE43 using the measured temperatures and heat flux transmitted through the sample at the steady state as follows:
Because the expanded char layer is a porous medium, the internal heat transfer includes both conduction and radiation. The thermal conduction occurs mainly through the solid phase, and the radiation takes place in the gas phase between the pore walls at different temperatures. If the internal heat transfer is treated as if conduction is the only mechanism, as a practical approximation, the apparent thermal conductivity (k) is determined using the measured temperatures and transmitted heat flux as follows:
where \(\dot{q}\) is the heat flux through the char layer, ΔT and Δx are the differences of the temperature and distance, respectively, between two thermocouple locations from the top surface of the steel plate (see Figure 1b). With this method, the temporal variations in the apparent thermal conductivity of expanding char layer are measured in situ under actual temperatures.
Figure 2 shows a water-based intumescent coating before and after the heat exposure. The white coating swelled ≈20 × in thickness by forming the porous char layer. The char layer was analyzed by a microcomputer tomography (CT) scanner to reveal the morphological structure and measure the porosity distribution (Figure 3). The top layer of coating seems to carbonize in the early stage to form a shell, which provides thermal insulation and sealing effect to the coating below. The lower layer of coating then starts to expand, when exceeding an activation temperature, by off-gassing from the carbonaceous–resinous mixture to form relatively large bubbles. The walls of the carbonized bubble structure seem to support the top-layer shell.

Reprinted with permission from Reference 42. © 2019 Springer Nature.
A water-based intumescent coating (a, b) before and (c, d) after the heat exposure. Incident heat flux, 50 kW/m2; initial coating thickness, 1.27 mm; char thickness, 27.9 mm.

Reprinted with permission from Reference 42. © 2019 Springer Nature.
(a) A photograph of an expanded water-based intumescent coating with a cross-sectional CT-scanned image (inset) and (b, c) 3D reconstructed char images from a region of interest (red rectangle). Incident heat flux, 75 kW/m2; initial coating thickness, 1.27 mm; and char thickness, 24.4 mm.
In this study, the three-dimensional void space (i.e., pores and cavities filled with air and some residual gases generated from chemical reactions) inside a char layer can be distinguished from the matter (carbonaceous char) simply by setting a threshold value. Thus, the void space and the solid are black and white, respectively, in the images (Figure 3a inset, b, c). Porosity (i.e., the fraction of the volume of void space in the total or bulk volume of material, including the solid and void components) is one of the key factors that directly link to a porous media’s thermal insulating performance.
Typical values of the measured thermal characteristics of the expanded char layer in response to the incident radiant flux of 25–75 kW/m2 are as follows: in-depth temperature (200–600°C), the heat-blocking efficiency (70–85%), the apparent thermal conductivity (0.2–0.3 W/mK), and the porosity (0.3–0.6).
As shown in Figures 2 and 3, the mass of the intumescent coating material initially contained in a thin layer expands ≈20 × to form a thick char layer with 30–60% of void space, thereby resulting in a good insulation (low apparent thermal conductivity). However, because of the porous structure, the char layer’s mechanical strength is generally weak. Therefore, materials to stabilize and retain the char are needed to assemble a multilayer thermal barrier.
Fire blanket materials
Fire blankets have been used for both active and passive fire protection (i.e., suppression and insulation). Despite their ease of handling compared to fire extinguishers, fire blankets have been used for smothering relatively small incipient fires only. They are generally not recommended to be used for a liquid fire or lab equipment as it can cause the fire to spread, although some products are claimed to be useable for cooking oil fires. There have been no adequate performance-based standards and ongoing third-party certification to those standards specifically designed for fire blankets. In early 2007, the American National Standards Institute adopted44 a performance-based standard for welding curtains, blankets, and pads. Fabrics used for hot work operations such as welding and cutting are also commonly known as fire blankets. The performance of fire blankets for protection of stored ammunition has also been studied.45,46,47,48
To contribute to firefighter and public safety by reducing the risk of building structure ignition, fire blankets for wrapping a whole house have been investigated43,49 in the laboratory and prescribed wildland fires. The fire blankets aim to prevent structure ignition by (1) blocking firebrands to enter homes through vulnerable spots (gutters, eaves, vents, broken windows, and roofs); (2) keeping homes from making direct contact with flames of surrounding combustibles (vegetation and mulch); and (3) reflecting thermal radiation from a large fire within close range (adjacent burning houses or surface-to-crown forest fires) for a sustained period of time.
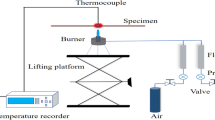
The thermal response characteristics of more than 50 relatively thin (0.15–3.7 mm) fire blanket materials from four different fiber groups (aramid, fiberglass, amorphous silica, and preoxidized carbon), and their composites have been investigated. Although the whole-house fire protection is orders-of-magnitude larger scale, these laboratory-scale results are still applicable to potential applications for Li-ion battery thermal runaway protection. A plain or coated fabric sample was subjected to a predominantly convective or radiant heat flux (up to 84 kW/m2) using a Meker burner (Figure 4) and a cone heater (not shown), respectively. The radiant cone heater apparatus is similar to the one shown in Figure 1, except that the heater radiates upward.
For most woven fabrics, the HBE values43 (Equation 1) were approximately 70 ± 10% for both convection and radiation and only mildly increased with the fabric thickness or the incident heat flux. Nonwoven (felt) fabrics with low thermal conductivity exhibited significantly better insulation (up to 87%) against convective heat. Highly reflective aluminum-coated materials exhibited exceptionally high HBE values (up to 98%) for radiation, whereas preoxidized carbon and charred aramid fabrics showed lower HBEs (down to 50%) due to efficient radiation absorption.43 In the laboratory experiment, two-layer thin fabric assemblies were able to block up to 92% of the convective heat and up to 96% of the radiation (with an aluminized surface).49
For a fixed thickness, the descending order of the HBE in the Meker burner (convective heat) is carbon (non-woven) > aramid/carbon/fiberglass > carbon (woven) > fiberglass ≈ aramid/fiberglass ≈ silica.
The descending order of the HBE in general correlates inversely with the following thermal conductivity data: aramid/carbon/fiberglass < fiberglass ≈ aramid/fiberglass < silica.
The relatively thin (1 mm order) fire blanket materials demonstrated the high heat-blocking efficiencies (60–90%), which are comparable to those of ≈20 mm-thick expanded intumescent coating char layer. Fire blankets are suitable not only for thermal insulation and smothering a small fire but also for direct-flame contact protection, flame penetration prevention, and jet-flame deflection. There are many commercial products of fire blanket materials on the market (e.g., Li-ion battery fire containment blankets, bags, and gloves).
The numerical simulations have also been performed50 to simulate the heat-transfer phenomena in the laboratory experiment based on the physics-based modeling using the one-dimensional transient heat-transfer equation, which includes radiation as well as conduction in the interior of layered fire blanket materials.
The explosive rupture of the cell case during a Li-ion battery fire can scatter high-temperature debris in every direction. To protect surroundings from damage and secondary fires, a fire blanket must withstand the ballistic impact as well as heat. The fire blankets for protection of stored ammunition45,46,47,48 requires both fire resistance and fragment penetration resistance. Textile fabrics in either soft or rigid forms are used for personal and equipment protection against ballistic and stab threats.51 The impact resistance of high-strength fabrics makes them desirable in applications such as protective clothing, protective layering in turbine fragment containment, and armor plating of vehicles.52 The use of specialized nanomaterials has been shown to improve the ballistic performance.51,53 Some of the high-strength fibers possess high melting temperature (>1200°C), thus, making them suitable for Li-ion battery thermal runaway protection and fire-protection applications under consideration.
Summary
The passive fire protection for failure resistance is needed as part of built-in safety mechanisms in commercial products using Li-ion batteries. Multilayer fire protective assemblies with components possessing different functions are desirable to resist failure of Li-ion batteries. Such multilayer fire protective assemblies can be made from intumescent coating materials, heat-blocking fire blankets, and soft or rigid impact-resistant high-strength fabrics for personal protective equipment, turbine fragment containment, and armor plating of vehicles. The layered assemblies can potentially be combined with heat-dissipating means, including metals (e.g., aluminum or copper), water, and phase-change materials. Successful multilayer assemblies can be used for various commercial products, including passive and active fire-protection blankets, battery-cell partitions, confinement bags and containers, packaging materials, and personal protective equipment.
Challenges in developing such fire protective systems against the cascading Li-ion battery failure may stem from the variability among various products. Although the basic principles for thermal insulation and heat dissipation remain the same, the requirements of the system performance, geometries, and, in turn, the choice of materials and designs depend on applications. Furthermore, these requirements will change with the progress in the battery technologies such as an anticipated increase in the energy storage capacity.
Naturally, these challenges direct the developmental efforts to customize the materials and design to specific applications. The compact lightweight designs are crucially important in applications in transportation (electric vehicles, aircraft, and spacecraft) as well as consumer electronics and devices, compared to high energy-storage capacity applications in grid energy and industry. More violent actions and severer damage expected from the higher energy capacity call for more active protection (i.e., fire suppression). Fire-extinguishing agents in the form of powder or gels, which generate inert gases or release chemical inhibitors, can be implemented into the protection systems. Finally, opportunities lie ahead for the comprehensive numerical modeling to understand the heat-blocking mechanisms and assist in designing the systems for the Li-ion battery failure resistance.
Data availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Change history
24 June 2021
A Correction to this paper has been published: https://doi.org/10.1557/s43577-021-00134-z
References
F. Larsson, B.-E. Mellander, Lithium-Ion Batteries Used in Electrified Vehicles—General Risk Assessment and Construction Guidelines from a Fire and Gas Release Perspective (RISE Research Institutes of Sweden, 2017)
Q. Wang, B. Mao, S.I. Stoliarov, J. Sun, Prog. Energy Combust. Sci. 73, 95 (2019)
T.O. Ely, D. Kamzabek, D. Chakraborty, Front. Energy Res. 7, 71 (2019)
D. Ouyang, D.M. Chen, Q. Huang, J. Weng, Z. Wang, J. Wang, Appl. Sci. 9, 2483 (2019)
P. Sun, X. Huang, R. Bisschop, H. Niu, Fire Technol. 56, 1361 (2020)
L.B. Diaz, X. He, Z. Hu, F. Restuccia, M. Marinescu, J.V. Barreras, Y. Patel, G. Offer, G. Rein, J. Electrochem. Soc. 167, 090559 (2020)
J. Sun, B. Mao, Q. Wang, Fire Saf. J. (2020). https://doi.org/10.1016/j.firesaf.2020.103119
D. Finegan, M. Scheel, J. Robinson, B. Tjaden, I. Hunt, T.J. Mason, J. Millichamp, M. DiMichiel, G.J. Offer, G. Hinds, D.J.L. Brett, P.R. Shearing, Nat. Commun. 6, 6924 (2015)
Y. Fu, S. Lu, K. Li, C. Liu, X. Cheng, H. Zhang, J. Power Sources 273, 216 (2015)
C.F. Lopez, J.A. Jeevarajan, P.P. Mukherjee, J. Electrochem. Soc. 162, A2163 (2015)
A.W. Golubkov, S. Scheikl, R. Planteu, G. Voitic, H. Wiltsche, C. Stangl, G. Fauler, A. Thalera, V. Hacker, RSC Adv. 5, 57171 (2015)
D.P. Finegan, E. Darcy, M. Keyser, B. Tjaden, T.M.M. Heenan, R. Jervis, J.J. Bailey, R. Malik, N.T. Vo, O.V. Magdysyuk, R. Atwood, M. Drakopoulos, M. DiMichiel, A. Rack, G. Hinds, D.J.L. Brett, P.R. Shearing, Energy Environ. Sci. 10, 1377 (2017)
D.P. Finegan, B. Tjaden, T.M.M. Heenan, R. Jervis, M. Di Michiel, A. Rack, G. Hinds, D.J.L. Brett, P.R. Shearing, J. Electrochem. Soc. 164(13), A3285 (2017)
B. Mao, H. Chen, Z. Cui, T. Wu, Q. Wang, Int. J. Heat Mass Transf. 122, 1103 (2018)
D.P. Finegan, J. Darst, W. Walker, Q. Li, C. Yang, R. Jervis, T.M.M. Heenan, J. Hack, J.C. Thomas, A. Rack, D.J.L. Brett, P.R. Shearing, M. Keyser, E. Darcy, J. Power Sources 417, 29 (2019)
S. Al-Hallaj, J.R. Selman, J. Power Sources 110, 341 (2002)
J. Lamb, C.J. Orendorff, L.A.M. Steele, S.W. Spangler, J. Power Sources 283, 517 (2015)
X. Feng, J. Sun, M. Ouyang, F. Wang, X. He, L. Lu, H. Peng, J. Power Sources 275, 261 (2015)
C.F. Lopez, J.A. Jeevarajan, P.P. Mukherjee, J. Electrochem. Soc. 162, A1905 (2015)
P. Huang, P. Ping, K. Li, H. Chen, Q. Wang, J. Wen, J. Sun, Appl. Energy 183, 659 (2016)
A.O. Said, C. Lee, S.I. Stoliarov, A.W. Marshall, Appl. Energy 248, 415 (2019)
M. Chen, O. Dongxu, J. Liu, J. Wang, Appl. Therm. Eng. 157, 113750 (2019)
D.P. Finegan, E. Darcy, M. Keyser, B. Tjaden, T.M.M. Heenan, R. Jervis, J.J. Bailey, N.T. Vo, O.V. Magdysyuk, M. Drakopoulos, M. Di Michiel, A. Rack, G. Hinds, D.J.L. Brett, P.R. Shearing, Adv. Sci. (2017). https://doi.org/10.1002/advs.201700369
F. Larsson, S. Bertilsson, M. Furlani, I. Albinsson, B.-E. Mellander, J. Power Sources 373, 220 (2018)
J. Chen, S. Kang, E. Jiaqiang, Z. Huang, K. Wei, B. Zhang, H. Zhu, Y. Deng, F. Zhang, G. Liao, J. Power Sources 442, 227228 (2019)
P. Goli, S. Legedza, A. Dhar, R. Salgado, J. Renteria, A.A. Balandin, J. Power Sources 248, 37 (2014)
Z.G. Qu, W.Q. Li, W.Q. Tao, Int. J. Hydrogen Energy 39, 3904 (2014)
Z. Ling, J. Chen, X. Fang, Z. Zhang, T. Xu, X. Gao, S. Wang, Appl. Energy 121, 104 (2014)
Y. Azizi, S.M. Sadrameli, Energy Convers. Manage. 128, 294 (2016)
S. Wilke, B. Schweitzer, S. Khateeb, S. Al-Hallaj, J. Power Sources 340, 51 (2017)
F. Larsson, J. Anderson, P. Andersson, B.-E. Mellander, J. Electrochem. Soc. 163, A2854 (2016)
P.T. Coman, E.C. Darcy, C.T. Veje, R.E. White, Appl. Energy 203(C), 189 (2017)
Q. Li, C. Yang, S. Santhanagopalan, K. Smith, J. Lamb, L.A. Steele, J. Power Sources 429, 80 (2019)
X. Yang, Y. Duan, Z. Zhang, X. Feng, T. Chen, C. Xu, X. Rui, M. Ouyang, L. Lu, X. Han, D. Ren, Z. Zhang, C. Li, S. Gao, Fire Technol. 56, 2579 (2020)
H.L. Vandersall, J. Fire Flammabl. 2, 97 (1971)
L.C. Camino, G. Martinasso, Polym. Degrad. Stab. 23, 359 (1989)
J. Hao, W.K. Chow, Archit. Sci. Rev. 46, 89 (2003)
E.D. Weil, J. Fire Sci. 29, 259 (2011)
T. Mariappan, J. Fire Sci. 34, 120 (2016)
R.G. Puri, A.S. Khanna, J. Coat. Technol. Res. 14, 1 (2017)
J. Kang, F. Takahashi, J.S. T’ien, J. Fire Sci. 36, 419 (2018)
J. Kang, F. Takahashi, J.S. T’ien, Fire Technol. 55, 689 (2019)
F. Takahashi, A. Abbott, T.M. Murray, J.S. T’ien, S.L. Olson, Fire Mater. 38, 609 (2014)
ANSI/FM 4950, American National Standard for Evaluating Welding Pads, Welding Blankets and Welding Curtains for Hot Work Operations (FM Approvals LLC, 2007)
W.K. Chin, T.J. Mulkern, A. Tewarson, Fire-Resistant and Fragment Penetration-Resistant Blankets for the Protection of Stored Ammunition (ARL-TR-2285, Army Research Laboratory, Aberdeen Proving Ground, MD, September 2000)
A. Tewarson, P.K. Wu, W.K. Chin, R. Shuford, Fire Blankets for Munition Protection: Flame and Heat Blocking Properties of Advanced Materials (ARL-TR-2398, Army Research Laboratory, February 2001)
B.J. Frame, J.G.R. Hansen, Thirty-First Department of Defense Explosives Safety Seminar Proceedings, San Antonio, TX, August 2004
J.G.R. Hansen, B.J. Frame, Fire Mater. 32, 457 (2008)
F. Takahashi, Front. Mech. Eng. 5, 60 (2019). https://doi.org/10.3389/fmech.2019.00060/full
S.-Y. Hsu, J.S. T’ien, F. Takahashi, S.L. Olson, Fire Safety Science: Proceedings of the Tenth International Symposium on Fire Safety Science (International Association for Fire Safety Science, Bethesda, MD, June 2011), 973–986. https://www.iafss.org/publications/fss/10/973#
K. Bilisik, Text. Res. J. 87, 2275 (2016)
A. Tabiei, G. Nilakantan, Appl. Mech. Rev. 61, 010801 (2008)
E.M. Soliman, M.P. Sheyka, M.R. Taha, Int. J. Impact Eng. 47, 39 (2012)
Acknowledgments
This work was supported in part by Underwriters Laboratories (UL) for the Intumescent Coatings Project and the US Department of Homeland Security, Federal Emergency Management Agency, Assistance to Firefighters Grant Program, Fire Prevention and Safety Grant No. EMW-2007-FP-02677, for the Fire Blanket Project. The author would like to thank J. Kang, Z.S. Fry, and co-op students for conducting the experiments; S.-Y. Hsu for performing the computation; J.S. T’ien and S.L. Olson for co-investigating the projects; and P. Gandhi, J.T. Chapin, and J.A. Jeevarajan for fruitful discussions.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author states that there is no conflict of interest.
Additional information
The original online version of this article was revised due to a retrospective Open Access order.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Takahashi, F. Fire blanket and intumescent coating materials for failure resistance. MRS Bulletin 46, 429–434 (2021). https://doi.org/10.1557/s43577-021-00102-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/s43577-021-00102-7