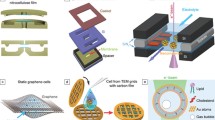
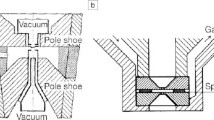
Abstract
Liquid cell transmission electron microscopy (TEM) has become an essential tool for studying the structure and properties of both hard and soft condensed-matter samples, as well as liquids themselves. Liquid cell sample holders, often consisting of two thin window layers separating the liquid sample from the high vacuum of the microscope column, have been designed to control in situ conditions, including temperature, voltage/current, or flow through the window region. While high-resolution and time-resolved TEM imaging probes the structure, shape, and dynamics of liquid cell samples, information about the chemical composition and spatially resolved bonding is often difficult to obtain due to the liquid thickness, the window layers, the holder configuration, or beam-induced radiolysis. In this article, we review different approaches to quantitative liquid cell electron microscopy, including recent developments to perform energy-dispersive x-ray and electron energy-loss spectroscopy experiments on samples in a liquid environment or the liquid itself. We also cover graphene liquid cells and other ultrathin window layer holders.




Similar content being viewed by others
References
L. Marton, Bull. Cl. Sci. Acad. R. Belg. 20, 439 (1934).
E. Ruska, Kolloid-Zeitschrift 100, 212 (1942).
I.M. Abrams, J.W. McBain, J. Appl. Phys. 15 607 (1944).
L. Marton, Rep. Prog. Phys. 10, 204 (1944).
F.M. Ross, J. Tersoff, M.C. Reuter, Phys. Rev. Lett. 95, 146104 (2005).
M.J. Williamson, R.M. Tromp, P.M. Vereecken, R. Hull, F.M. Ross, Nat. Mater. 2, 532 (2003).
L.F. Allard, W.C. Bigelow, M. Jose-Yacaman, D.P. Nackashi, J. Damiano, S.E. Mick, Microsc. Res. Tech. 72, 208 (2009).
H. Zheng, R.K. Smith, Y.W. Jun, C. Kisielowski, U. Dahmen, A.P. Alivisatos, Science 324, 1309 (2009).
B.L. Mehdi, J. Qian, E. Nasybulin, C. Park, D.A. Welch, R. Faller, H. Mehta, W.A. Henderson, W. Xu, C.M. Wang, J.E. Evans, J. Liu, J.G. Zhang, K.T. Mueller, N.D. Browning, Nano Lett. 15, 2168 (2015).
S. Keskin, N. de Jonge, Nano Lett. 18, 7435 (2018).
H. Cho, M.R. Jones, S.C. Nguyen, M.R. Hauwiller, A. Zettl, A.P. Alivisatos, Nano Lett. 17, 414 (2017).
M.E. Holtz, Y. Yu, J. Gao, H.D. Abruna, D.A. Muller, Microsc. Microanal. 19, 1027 (2013).
R.R. Unocic, L. Baggetto, G.M. Veith, J.A. Agular, K.A. Unocic, R.L. Sacci, N.J. Dudney, K.L. More, Chem. Commun. 51, 16377 (2015).
E.A. Lewis, S.J. Haigh, T.J. Slater, Z. He, M.A. Kulzick, M.G. Burke, N.J. Zaluzec, Chem. Commun. 50, 10019 (2014).
C. Wang, T. Shokuhfar, R.F. Klie, Adv. Mater. 28, 7716 (2016).
M.R. Hauwiller, J.C. Ondry, C.M. Chan, P. Khandekar, J. Yu, A.P. Alivisatos, J. Am. Chem. Soc. 141, 4428 (2019).
M.A. Aronova, R.D. Leapman, MRS Bull. 37, 53 (2012).
J.M. Yuk, K. Kim, B. Aleman, W. Regan, J.H. Ryu, J. Park, P. Ercius, H.M. Lee, A.P. Alivisatos, M.F. Crommie, J.Y. Lee, A. Zettl, Nano Lett. 11, 3290 (2011).
J.M. Yuk, J. Park, P. Ercius, K. Kim, D.J. Hellebusch, M.F. Crommie, J.Y. Lee, A. Zettl, A.P. Alivisatos, Science 336, 61 (2012).
D.J. Kelly, M. Zhou, N. Clark, M.J. Hamer, E.A. Lewis, A.M. Rakowski, S.J. Haigh, R.V. Gorbachev, Nano Lett. 18, 1168 (2018).
J. Park, H. Elmlund, P. Ercius, J.M. Yuk, D.T. Limmer, Q. Chen, K. Kim, S.H. Han, D.A. Weitz, A. Zettl, A.P. Alivisatos, Science 349, 290 (2015).
B.H. Kim, J. Heo, S. Kim, C.F. Reboul, H. Chun, D. Kang, H. Bae, H. Hyun, J. Lim, H. Lee, B. Han, T. Hyeon, A.P. Alivisatos, P. Ercius, H. Elmlund, J. Park, Science 368, 60 (2020).
J.M. Yuk, J. Park, P. Ercius, K. Kim, D.J. Hellebusch, M.F. Crommie, J.Y. Lee, A. Zettl, A.P. Alivisatos, Science 336, 61 (2012).
J.M. Yuk, H.K. Seo, J.W. Choi, J.Y. Lee, ACS Nano 8, 7478 (2014).
A. de Clercq, W. Dachraoui, O. Margeat, K. Pelzer, C.R. Henry, S. Giorgio, J. Phys. Chem. Lett. 5, 2126 (2014).
C. Wang, Q. Qiao, T. Shokuhfar, R.F. Klie, Adv. Mater. 26, 3410 (2014).
A.J. Donovan, J. Kalkowski, M. Szyrnusiak, C.H. Wang, S.A. Smith, R.F. Klie, J.H. Morrissey, Y. Liu, Biomacromolecules 17, 2572 (2016).
A.R. Ribeiro, A. Mukherjee, X. Hu, S. Shafien, R. Ghodsi, K. He, S. Gemini-Piperni, C. Wang, R.F. Klie, T. Shokuhfar, R. Shahbazian-Yassar, R. Borojevic, L.A. Rocha, J.M. Granjeiro, Nanoscale 9, 10684 (2017).
K.H. Nagamanasa, H. Wang, S. Granick, Adv. Mater. 29, 1703555 (2017).
J.R. Jokisaari, J.A. Hachtel, X. Hu, A. Mukherjee, C. Wang, A. Konecna, T.C. Lovejoy, N. Dellby, J. Aizpurua, O.L. Krivanek, J.-C. Idrobo, R.F. Klie, Adv. Mater. 30, 1802702 (2018).
H. Wang, B. Li, Y.-J. Kim, O.-H. Kwon, S. Granick, Proc. Natl. Acad. Sci. U.S.A. 117, 1283 (2020).
D.J. Banner, E. Firlar, J. Jakubonis, Y. Baggia, J.K. Osborn, R. Shahbazian-Yassar, C.M. Megaridis, T. Shokuhfar, Int. J. Nanomed. 15, 1929 (2020).
W. Zhou, K. Yin, C. Wang, Y. Zhang, T. Xu, A. Borisevich, L. Sun, J.C. Idrobo, M.F. Chisholm, S.T. Pantelides, R.F. Klie, A.R. Lupini, Nature 528, E1 (2015).
D. Shin, J.B. Park, Y.-J. Kim, S.J. Kim, J.H. Kang, B. Lee, S.-P. Cho, B.H. Hong, K.S. Novoselov, Nat. Commun. 6, 6068 (2015).
J. Yang, S.B. Alam, L. Yu, E. Chan, H. Zheng, Micron 116, 22 (2019).
P. Rez, T. Aoki, K. March, D. Gur, O.L. Krivanek, N. Dellby, T.C. Lovejoy, S.G. Wolf, H. Cohen, Nat. Commun. 7, 10945 (2016).
P.A. Crozier, Ultramicroscopy 180, 104 (2017).
J.A. Hachtel, A.R. Lupini, J.C. Idrobo, Sci. Rep. 8, 5637 (2018).
R.F. Egerton, Electron Energy Loss Spectroscopy in the Electron Microscope, 2nd ed. (Springer Science and Business Media, New York, 2011).
O.L. Krivanek, N. Dellby, J.A. Hachtel, J.C. Idrobo, M.T. Hotz, B. Plotkin-Swing, N.J. Bacon, A.L. Bleloch, G.J. Corbin, M.V. Hoffman, C.E. Meyer, T.C. Lovejoy, Ultramicroscopy 203, 60 (2019).
O.L. Krivanek, T.C. Lovejoy, N. Dellby, T. Aoki, R.W. Carpenter, P. Rez, E. Soignard, J.T. Zhu, P.E. Batson, M.J. Lagos, R.F. Egerton, P.A. Crozier, Nature 514, 209 (2014).
P.A. Crozier, T. Aoki, Q. Liu, Ultramicroscopy 169, 30 (2016).
D.M. Haiber, P.A. Crozier, ACS Nano 12, 5463 (2018).
J.A. Hachtel, J. Huang, I. Popovs, S. Jansone-Popova, J.K. Keum, J. Jakowski, T.C. Lovejoy, N. Dellby, O.L. Krivanek, J.C. Idrobo, Science 363, 525 (2019).
J.A. Hachtel, A.R. Lupini, J.C. Idrobo, Sci. Rep. 8, 5637 (2018).
M. Battaglia, D. Contarato, P. Denes, D. Doering, P. Giubilato, T.S. Kim, S. Mattiazzo, V. Radmilovic, S. Zalusky, Nucl. Instrum. Methods Phys. Res. A 598, 642 (2009).
P. Grob, D. Bean, D. Typke, X. Li, E. Nogales, R.M. Glaeser, Ultramicroscopy 133, 1 (2013).
M. Battaglia, D. Contarato, P. Denes, P. Giubilato, Nucl. Instrum. Methods Phys. Res. A 608, 363 (2009).
E. Nogales, Nat. Methods 13, 24 (2016).
X. Li, P. Mooney, S. Zheng, C.R. Booth, M.B. Braunfeld, S. Gubbens, D.A. Agard, Y. Cheng, Nat. Methods 10, 584 (2013).
C. Ophus, Microsc. Microanal. 25, 563 (2019).
M.J. Zachman, Z. Tu, S. Choudhury, L.A. Archer, L.F. Kourkoutis, Nature 560, 345 (2018).
H. Zheng, R.K. Smith, Y.-w. Jun, C. Kisielowski, U. Dahmen, A.P. Alivisatos , Science 324, 1309 (2009).
Y. Xie, S. Sohn, M. Wang, H. Xin, Y. Jung, M.D. Shattuck, C.S. O'Hern, J. Schroers, J.J. Cha, Nat. Commun. 10, 915 (2019).
J. Ciston, I.J. Johnson, B.R. Draney, P. Ercius, E. Fong, A. Goldschmidt, J.M. Joseph, J.R. Lee, A. Mueller, C. Ophus, A. Selvarajan, D.E. Skinner, T. Stezelberger, C.S. Tindall, A.M. Minor, P. Denes, Microsc. Microanal. 25, 1930 (2019).
R.F. Egerton, Micron 119, 72 (2019).
N. de Jonge, F.M. Ross, Nat. Nanotechnol. 6, 695 (2011).
N. de Jonge, D.B. Peckys, G.J. Kremers, D.W. Piston, Proc. Natl. Acad. Sci. U.S.A. 106, 2159 (2009).
H. Cho, M.R. Jones, S.C. Nguyen, M.R. Hauwiller, A. Zettl, A.P. Alivisatos, Nano Lett. 17, 414 (2017).
S. Keskin, N. de Jonge, Nano Lett. 18, 7435 (2018).
Q. Chen, J.M. Smith, H.I. Rasool, A. Zettl, A.P. Alivisatos, Faraday Discuss. 175, 203 (2014).
J. Park, H. Park, P. Ercius, A.F. Pegoraro, C. Xu, J.W. Kim, S.H. Han, D.A. Weitz, Nano Lett. 15, 4737 (2015).
R.F. Egerton, Ultramicroscopy 180, 115 (2017).
F.S. Hage, D.M. Kepaptsoglou, Q.M. Ramasse, L.J. Allen, Phys. Rev. Lett. 122, 016103 (2019).
K. Venkatraman, B.D.A. Levin, K. March, P. Rez, P.A. Crozier, Nat. Phys. 15, 1237 (2019).
F.S. Hage, G. Radtke, D.M. Kepaptsoglou, M. Lazzeri, Q.M. Ramasse, Science 367, 1124 (2020).
P.J. Gomes, A.M. Ferraria, A.M. Botelho do Rego, S.V. Hoffmann, P.A. Ribeiro, M. Raposo, J. Phys. Chem. B 119, 5404 (2015).
P. Rez, T. Aoki, K. March, D. Gur, O.L. Krivanek, N. Dellby, T.C. Lovejoy, S.G. Wolf, H. Cohen, Nat. Commun. 7, 10945 (2016).
F.M. Ross, Liquid Cell Electron Microscopy (Cambridge University Press, Cambridge, UK, 2016).
J.R. Dwyer, M. Harb, Appl. Spectrosc. 71, 2051 (2017).
P. Walde, S. Ichikawa, Biomol. Eng 18, 143 (2001).
D.T. Chiu, C.F. Wilson, F. Ryttsen, A. Stromberg, C. Farre, A. Karlsson, S. Nordholm, A. Gaggar, B.P. Modi, A. Moscho, R.A. Garza-Lopez, O. Orwar, R.N. Zare, Science 283, 1892 (1999).
S.M. Hoppe, D.Y. Sasaki, A.N. Kinghorn, K. Hattar, Langmuir 29, 9958 (2013).
J. Yang, M.K. Choi, Y. Sheng, J. Jung, K. Bustillo, T. Chen, S.-W. Lee, P. Ercius, J.H. Kim, J.H. Warner, E.M. Chan, H. Zheng, Nano Lett. 19, 1788 (2019).
J. Lindner, P. Vöhringer, M.S. Pshenichnikov, D. Cringus, D.A. Wiersma, M. Mostovoy, Chem. Phys. Lett. 421, 329 (2006).
H. Nagae, M. Kuki, J.-P. Zhang, T. Sashima, Y. Mukai, Y. Koyama, J. Phys. Chem. A 104, 4155 (2000).
P.R. Tulip, S.J. Clark, Phys. Rev. B 71, 195117 (2005).
H. Terrones, E.D. Corro, S. Feng, J.M. Poumirol, D. Rhodes, D. Smirnov, N.R. Pradhan, Z. Lin, M.A.T. Nguyen, A.L. Elías, T.E. Mallouk, L. Balicas, M.A. Pimenta, M. Terrones, Sci. Rep. 4, 4215 (2014).
Acknowledgments
The authors would like to thank J.R. Jokisaari for his help with Figure 2. P.E. is supported by the Molecular Foundry, Lawrence Berkeley National Laboratory, which is supported by the US Department of Energy (DOE) under Contract No. DE-AC02–05CH11231. P.E. thanks support from the DOE Office of Science, Office of Basic Energy Sciences, Materials Sciences and Engineering Division under Contract No. DE-AC02–05-CH11231 within the KC22ZH program. R.F.K. is supported in part by the Joint Center for Energy Storage Research (JCESR), an energy innovation hub funded by the US DOE, Office of Science, Basic Energy Sciences. J.A.H.’s portion of this work was supported by the Center for Nanophase Materials Sciences, which is a DOE Office of Science User Facility.
Appendix
Appendix
 Peter Ercius is a staff scientist at the National Center for Electron Microscopy (NCEM) facility within the Molecular Foundry Division at Lawrence Berkeley National Laboratory. He is the main staff contact for the TEAM 0.5 aberration-corrected instrument. He received his BS degree in 2003, and his PhD degree in 2009 in applied and engineering physics from Cornell University. He completed postdoctoral research at the NCEM before being hired as a staff scientist. His research focuses on atomic-resolution electron tomography, scanning nanodiffraction (4D-scanning transmission electron microscopy), in situ liquid cell electron microscopy, and 2D/3D image analysis. Ercius can be reached by email at percius@lbl.gov.
Peter Ercius is a staff scientist at the National Center for Electron Microscopy (NCEM) facility within the Molecular Foundry Division at Lawrence Berkeley National Laboratory. He is the main staff contact for the TEAM 0.5 aberration-corrected instrument. He received his BS degree in 2003, and his PhD degree in 2009 in applied and engineering physics from Cornell University. He completed postdoctoral research at the NCEM before being hired as a staff scientist. His research focuses on atomic-resolution electron tomography, scanning nanodiffraction (4D-scanning transmission electron microscopy), in situ liquid cell electron microscopy, and 2D/3D image analysis. Ercius can be reached by email at percius@lbl.gov.
 Jordan Hachtel has been a staff scientist at the Center for Nanophase Materials Sciences at Oak Ridge National Laboratory (ORNL) since 2019. He received his PhD degree in physics from Vanderbilt University in 2016. He completed postdoctoral research at ORNL. His research focuses on ultrahigh-energy resolution monochromated electron energy-loss spectroscopy analysis of infrared phonons, plasmons, polaritons, and molecular vibrations in a high-spatial resolution aberration-corrected scanning transmission electron microscopy. Hachtel can be reached by email at hachtelja@ornl.gov.
Jordan Hachtel has been a staff scientist at the Center for Nanophase Materials Sciences at Oak Ridge National Laboratory (ORNL) since 2019. He received his PhD degree in physics from Vanderbilt University in 2016. He completed postdoctoral research at ORNL. His research focuses on ultrahigh-energy resolution monochromated electron energy-loss spectroscopy analysis of infrared phonons, plasmons, polaritons, and molecular vibrations in a high-spatial resolution aberration-corrected scanning transmission electron microscopy. Hachtel can be reached by email at hachtelja@ornl.gov.
 Robert F. Klie is a professor of physics at the University of Illinois at Chicago (UIC). He received his PhD degree from UIC in 2002. Between 2002–2005, he was a Goldhaber Fellow at Brookhaven National Laboratory. His research includes in situ characterization of materials using aberration-corrected scanning transmission electron microscopy and electron spectroscopies. His current research focuses on novel approaches of electron energy-loss spectroscopy (EELS) to study materials, including nanoscale thermometry using low-loss EELS, or two-dimensional layer liquid cell to characterize water, biological systems, and solid–liquid interfaces. Klie can be reached by email at rfklie@uic.edu.
Robert F. Klie is a professor of physics at the University of Illinois at Chicago (UIC). He received his PhD degree from UIC in 2002. Between 2002–2005, he was a Goldhaber Fellow at Brookhaven National Laboratory. His research includes in situ characterization of materials using aberration-corrected scanning transmission electron microscopy and electron spectroscopies. His current research focuses on novel approaches of electron energy-loss spectroscopy (EELS) to study materials, including nanoscale thermometry using low-loss EELS, or two-dimensional layer liquid cell to characterize water, biological systems, and solid–liquid interfaces. Klie can be reached by email at rfklie@uic.edu.
Rights and permissions
About this article
Cite this article
Ercius, P., Hachtel, J.A. & Klie, R.F. Chemical and bonding analysis of liquids using liquid cell electron microscopy. MRS Bulletin 45, 761–768 (2020). https://doi.org/10.1557/mrs.2020.230
Published:
Issue Date:
DOI: https://doi.org/10.1557/mrs.2020.230