Abstract
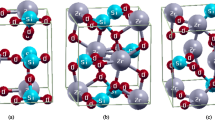
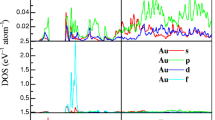
In this work, using density functional theory we study electronic and atomic structure as well as redistribution of ions at the interface between cubic-Li7La3Zr2O12 (LLZO) (001) and LiCoO2 (LCO) (10-14). It is found that a large lattice-mismatch-induced compressive strain of ~12% at the interface leads to disordering of LLZO (001). However, even a large tensile strain of ~13.5% does not influence ordering of LCO (10-14). Li ions tend to move from the surface of LCO and bulk LLZO to occupy the interstitial sites at the topmost layers of the LLZO slab. Li ion transfer from LCO to LLZO accompanies with electron transfer from the former to the latter and the formation of gap states.



Similar content being viewed by others
References
C. Masquelier: Solid electrolytes: lithium ions on the fast track. Nat. Mater. 10, 649 (2011).
J-M. Tarascon and M. Armand: Issues and challenges facing rechargeable lithium batteries. Nature 414(6861), 359–367 (2001).
J. Hassoun and B. Scrosati: Moving to a solid-state configuration: a valid approach to making lithium-sulfur batteries viable for practical applications. Adv. Mater. 22, 5198–5201 (2010).
H. Zhong, C. Wang, Z. Xu, F. Ding, and X. Liu: A novel quasi-solid state electrolyte with highly effective polysulfide diffusion inhibition for lithium-sulfur batteries. Sci. Rep. 6, 25484 (2016).
V. Thangadurai, H. Kaack, and W. Weppner: Novel fast lithium ion conduction in garnet-type Li5La3M2O12 (M = Nb, Ta). J. Am. Ceram. Soc. 86, 437–440 (2003).
V. Thangadurai and W. Weppner: Li6ALa2Ta2O12 (A = Sr, Ba): novel garnet-like oxides for fast lithium ion conduction. Adv. Funct. Mater. 15, 107–112 (2005).
V. Thangadurai and W. Weppner: Li6ALa2Nb2O12 (A = Ca, Sr, Ba): a new class of fast lithium ion conductors with garnet-like structure. J. Am. Ceram. Soc. 88, 411–418 (2005).
R. Murugan, V. Thangadurai, and W. Weppner: Fast lithium ion conduction in garnet-type Li7La3Zr2O12. Angew. Chem., Int. Ed. 46, 7778–7781 (2007).
J. Awaka, A. Takashima, K. Kataoka, N. Kijima, Y. Idemoto, and J. Akimoto: Crystal structure of fast lithium-ion-conducting cubic Li7La3Zr2O12. Chem. Lett. 40, 60–62 (2010).
Y. Shimonishi, A. Toda, T. Zhang, A. Hirano, N. Imanishi, O. Yamamoto, and Y. Takeda: Synthesis of garnet-type Li7-xLa3Zr2O12-1/2x and its stability in aqueous solutions. Solid State Ion. 183, 48–53 (2011).
R. Murugan, S. Ramakumar, and N. Janani: High conductive yttrium doped Li7La3Zr2O12 cubic lithium garnet. Electrochem. Commun. 13, 1373–1375 (2011).
Y. Wang and W. Lai: High ionic conductivity lithium garnet oxides of Li7-xLa3Zr2-xTaxO12 compositions. Electrochem. Solid-State Lett. 15, A68–A71 (2012).
Y. Zhu, X. He, and Y. Mo: Origin of outstanding stability in the lithium solid electrolyte materials: insights from thermodynamic analyses based on first-principles calculations. ACS Appl. Mater. Interfaces 7, 23685–23693 (2015).
Y. Zhu, X. He, and Y. Mo: First principles study on electrochemical and chemical stability of solid electrolyte-electrode interfaces in all-solid-state Li-ion batteries. J. Mater. Chem. A 4, 3253–3266 (2016).
T. Thompson, S. Yu, L. Williams, R.D. Schmidt, R. Garcia-Mendez, J. Wolfenstine, J.L. Allen, E. Kioupakis, D.J. Siegel, and J. Sakamoto: Electrochemical window of the Li-ion solid electrolyte Li7La3Zr2O12. ACS Energy Lett. 2, 462–468 (2017).
R. Koksbang, J. Barker, H. Shi, and M.Y. Saidi: Cathode materials for lithium rocking chair batteries. Solid State Ion. 84, 1–21 (1996).
K. Mizushima, P.C. Jones, P.J. Wiseman, and J.B. Goodenough: LixCoO2 (0 > x = 1): a new cathode material for batteries of high energy density. Solid State Ion. 3, 171–174 (1981).
K. Mizushima, P.C. Jones, P.J. Wiseman, and J.B. Goodenough: LixCoO2 (0 > x>-1): a new cathode material for batteries of high energy density. Mater. Res. Bull. 15, 783–789 (1980).
T. Ohzuku and A. Ueda: Why transition metal (di) oxides are the most attractive materials for batteries. Solid State Ion. 69, 201–211 (1994).
A. Du Pasquier, I. Plitz, S. Menocal, and G. Amatucci: A comparative study of Li-ion battery, supercapacitor and nonaqueous asymmetric hybrid devices for automotive applications. J. Power Sources 115, 171–178 (2003).
Y. Ren, T. Liu, Y. Shen, Y. Lin, and C-W. Nan: Chemical compatibility between garnet-like solid state electrolyte Li6.75La3Zr1.75Ta0.25O12 and major commercial lithium battery cathode materials. J. Materiomics 2, 256–264 (2016).
K. Takada, N. Ohta, L. Zhang, K. Fukuda, I. Sakaguchi, R. Ma, M. Osada, and T. Sasaki: Interfacial modification for high-power solid-state lithium batteries. Solid State Ion. 179, 1333–1337 (2008).
K.H. Kim, Y. Iriyama, K. Yamamoto, S. Kumazaki, T. Asaka, K. Tanabe, C.A.J. Fisher, T. Hirayama, R. Murugan, and Z. Ogumi: Characterization of the interface between LixCoO2 and Li7La3Zr2O12 in an all-solid-state rechargeable lithium battery. J. Power Sources 196, 764–767 (2011).
K. Park, B-C. Yu, J-W. Jung, Y. Li, W. Zhou, H. Gao, S. Son, and J.B. Goodenough: Electrochemical nature of the cathode interface for a solid-state lithium-ion battery: interface between LiCoO2 and Garnet-Li7La3Zr2O12. Chem. Mater. 28, 8051–8059 (2016).
M. Sumita, Y. Tanaka, M. Ikeda, and T. Ohno: Theoretically designed Li3PO4(100)/LiFePO4(010) coherent electrolyte/cathode interface for all solid-state Li ion secondary batteries. J. Phys. Chem. C 119, 14–22 (2014).
M. Sumita, Y. Tanaka, M. Ikeda, and T. Ohno: Charged and discharged states of cathode/sulfide electrolyte interfaces in all-solid-state lithium ion batteries. J. Phys. Chem. C 120, 13332–13339 (2016).
J. Haruyama, K. Sodeyama, L. Han, K. Takada, and Y. Tateyama: Space-charge layer effect at interface between oxide cathode and sulfide electrolyte in all-solid-state lithium-ion battery. Chem. Mater. 26, 4248–4255 (2014).
G. Lippert, J. Hutter, and M. Parrinello: A hybrid Gaussian and plane wave density functional scheme. Mol. Phys. 92, 477–488 (1997).
G. Lippert, J. Hutter, and M. Parrinello: The Gaussian and augmented-plane-wave density functional method for ab initio molecular dynamics simulations. Theor. Chem. Acc. (Theoret. Chim. Acta) 103, 124–140 (1999).
J. VandeVondele, and J. Hutter: An efficient orbital transformation method for electronic structure calculations. J. Chem. Phys. 118, 4365–4369 (2003).
J. VandeVondele, M. Krack, F. Mohamed, M. Parrinello, T. Chassaing, and J. Hutter: Quickstep: fast and accurate density functional calculations using a mixed Gaussian and plane waves approach. Comput. Phys. Commun. 167, 103–128 (2005).
J.P. Perdew, K. Burke, and M. Ernzerhof: Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865 (1996).
S.L. Dudarev, G.A. Botton, S.Y. Savrasov, C.J. Humphreys, and A.P. Sutton: Electron-energy-loss spectra and the structural stability of nickel oxide: an LSDA + U study. Phys. Rev. B 57, 1505 (1998).
P. Ghosh, S. Mahanty, M.W. Raja, R.N. Basu, and H.S. Maiti: Structure and optical absorption of combustion-synthesized nanocrystalline LiCoO2. J. Mater. Res. 22, 1162–1167 (2007).
K. Kushida and K. Kuriyama: Narrowing of the co-3d band related to the order-disorder phase transition in LiCoO2. Solid State Commun.. 123, 349–352 (2002).
J.M. Rosolen and F. Decker: Photoelectrochemical behavior of LiCoO2 membrane electrode. J. Electroanal. Chem. 501, 253–259 (2001).
J. Van Elp, J.L. Wieland, H. Eskes, P. Kuiper, G.A. Sawatzky, F.M.F. De Groot, and T.S. Turner: Electronic structure of coo, Li-doped coo, and LiCoO2. Phys. Rev. B 44, 6090 (1991).
L.J. Miara, S.P. Ong, Y. Mo, W.D. Richards, Y. Park, J-M. Lee, H.S. Lee, and G. Ceder: Effect of Rb and Ta doping on the ionic conductivity and stability of the garnet Li7+2x-y(La3-xRbx)(Zr2-yTay)O12 (0 = x = 0.375, 0 = y = 1) superionic conductor: a first principles investigation. Chem. Mater. 25, 3048–3055 (2013).
K.C. Santosh, R.C. Longo, K. Xiong, and K. Cho: Point defects in garnet-type solid electrolyte (c-Li7La3Zr2O12) for Li-ion batteries. Solid State Ion. 261, 100–105 (2014).
R. Jalem, Y. Yamamoto, H. Shiiba, M. Nakayama, H. Munakata, T. Kasuga, and K. Kanamura: Concerted migration mechanism in the Li ion dynamics of garnet-type Li7La3Zr2O12. Chem. Mater. 25, 425–430 (2013).
L.J. Miara, W.D. Richards, Y.E. Wang, and G. Ceder: First-principles studies on cation dopants and electrolyte-cathode interphases for lithium garnets. Chem. Mater. 27, 4040–4047 (2015).
K. Meier, T. Laino, and A. Curioni: Solid-state electrolytes: revealing the mechanisms of Li-ion conduction in tetragonal and cubic LLZO by first-principles calculations. J. Phys. Chem. C 118, 6668–6679 (2014).
S. Goedecker, M. Teter, and J. Hutter: Separable dual-space Gaussian pseudopotentials. Phys. Rev. B 54, 1703 (1996).
D. Ensling, A. Thissen, S. Laubach, P.C. Schmidt, and W. Jaegermann: Electronic structure of LiCoO2 thin films: a combined photoemission spectroscopy and density functional theory study. Phys. Rev. B 82, 195431 (2010).
I. Rodrigues, J. Wontcheu, and D.D. MacNeil: Post-synthetic treatments on NixMnxCo1-2x(OH)2 for the preparation of lithium metal oxides. Mater. Res. Bull. 46, 1878–1886 (2011).
S. Panahian Jand and P. Kaghazchi: The role of electrostatic effects in determining the structure of LiF-graphene interfaces. J. Phys.: Condens. Matter 26, 262001 (2014).
M. Okubo, E. Hosono, J. Kim, M. Enomoto, N. Kojima, T. Kudo, H. Zhou, and I. Honma: Nanosize effect on high-rate Li-ion intercalation in LiCoO2 electrode. J. Am. Chem. Soc. 129, 7444–7452 (2007).
D. Kramer and G. Ceder: Tailoring the morphology of LiCoO2: a first principles study. Chem. Mater. 21, 3799–3809 (2009).
Acknowledgment
The authors gratefully acknowledge support from the “Bundesministerium für Bildung und Forschung” (BMBF), and the computing time granted on Zentraleinrichtung für Datenverarbeitung (ZEDAT) at the Freie Universität Berlin. The authors also acknowledge the North-German Supercomputing Alliance (HLRN) for providing high-performance computing resources that have contributed to the research results reported in this paper.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jand, S.P., Kaghazchi, P. Theoretical study of cubic-Li7La3Zr2O12(001)/LiCoO2(10-14) interface. MRS Communications 8, 591–596 (2018). https://doi.org/10.1557/mrc.2018.33
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/mrc.2018.33