Abstract
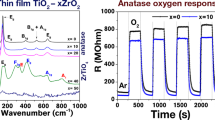
Titanium dioxide (TiO2) is a semiconductor that can be applied in different technological areas. In this work, we investigated the modifications of the electrical properties of thin films composed of TiO2 nanoparticles produced with different morphologies. The solvothermal route used for the synthesis allowed the production of nanoparticles with functionalized surfaces due to oleate groups. It was possible to modulate nanocrystals shape and size due to the detachment crystal growth mechanism, by changing the reaction time. Nanorods were obtained using 4 h of synthesis, and an increase in the reaction time to 64 h led to a bipyramidal morphology. The functionalization by the organic ligand allowed the preparation of stable colloidal solutions, which were used to prepare thin films by the dip-coating method. The films presented a homogeneous surface, an average thickness around 100 nm, and no agglomerations were observed. The electrical resistance measurements indicated a typical behavior of semiconductors, and they were dependent on the nanoparticle morphology. An exploratory test indicated that the thin films prepared using nanorod particles presented a higher electrical response compared with isotropic particles, when exposed in a liquefied petroleum gas vapor atmosphere. Therefore, the morphology of the nanoparticles is a key factor for the further application of these thin films in gas sensing. Employing an easy methodology which required simple apparatus, and by using reaction time modulation only, it was possible to prepare homogeneous thin films with a tunable electrical response.










Similar content being viewed by others
References
M.S. Ahmad, A.K. Pandey, and N.A. Rahim: Advancements in the development of TiO2 photoanodes and its fabrication methods for dye sensitized solar cell (DSSC) applications. A review. Renew. Sustain. Energy Rev. 77, 89 (2017).
L. Xu, J. Xu, H. Hu, C. Cui, Z. Ding, Y. Yan, P. Lin, and P. Wang: Hierarchical submicroflowers assembled from ultrathin anatase TiO2 nanosheets as light scattering centers in TiO2 photoanodes for dye-sensitized solar cells. J. Alloys Compd. 776, 1002 (2019).
G. Kenanakis, D. Vernardou, A. Dalamagkas, and N. Katsarakis: Photocatalytic and electrooxidation properties of TiO2 thin films deposited by sol–gel. Catal. Today 240, 146 (2015).
J. Singh, S.A. Khan, J. Shah, R. Kotnala, and S. Mohapatra: Nanostructured TiO2 thin films prepared by RF magnetron sputtering for photocatalytic applications. Appl. Surf. Sci. 422, 953 (2017).
C. Lin, Y. Gao, J. Zhang, D. Xue, H. Fang, J. Tian, C. Zhou, C. Zhang, Y. Li, and H. Li: GO/TiO2 composites as a highly active photocatalyst for the degradation of methyl orange. J. Mater. Res. 35, 1307 (2020).
N.T.T. Thuy, D.H. Tung, L.H. Manh, J.H. Kim, S.I. Kudryashov, and P.H.J.A.S. Minh: Plasma enhanced wet chemical surface activation of TiO2 for the synthesis of high performance photocatalytic Au/TiO2 nanocomposites. Appl. Sci. 10, 3345 (2020).
V. Galstyan: Porous TiO2-based gas sensors for cyber chemical systems to provide security and medical diagnosis. Sensors 17, 2947 (2017).
X. Li, Y. Zhao, X. Wang, J. Wang, A.M. Gaskov, and S. Akbar: Reduced graphene oxide (rGO) decorated TiO2 microspheres for selective room-temperature gas sensors. Sens. Actuat., B 230, 330 (2016).
J. Bai and B. Zhou: Titanium dioxide nanomaterials for sensor applications. Chem. Rev. 114, 10131 (2014).
E. Bayan, T. Lupeiko, L. Pustovaya, M. Volkova, V. Butova, and A. Guda: Zn–F co-doped TiO2 nanomaterials: Synthesis, structure and photocatalytic activity. J. Alloys Compd. 822, 153662 (2020).
J. Huang and Q. Wan: Gas sensors based on semiconducting metal oxide one-dimensional nanostructures. Sensors 9, 9903 (2009).
G. Korotcenkov: Metal oxides for solid-state gas sensors: What determines our choice? Mater. Sci. Eng., B 139, 1 (2007).
C. Wang, L. Yin, L. Zhang, D. Xiang, and R. Gao: Metal oxide gas sensors: Sensitivity and influencing factors. Sensors 10, 2088 (2010).
X. Chen and S.S. Mao: Titanium dioxide nanomaterials: Synthesis, properties, modifications, and applications. Chem. Rev. 107, 2891 (2007).
D.-S. Lee, D.-D. Lee, S.-W. Ban, M. Lee, and Y.T. Kim: SnO2 gas sensing array for combustible and explosive gas leakage recognition. IEEE Sens. J. 2, 140 (2002).
K.R. Nemade, R.V. Barde, and S.A. Waghuley: Liquefied petroleum gas sensing by Al-doped TiO2 nanoparticles synthesized by chemical and solid-state diffusion routes. J. Taibah Univ. Sci. 10, 345 (2015).
N. Liu, X. Chen, J. Zhang, and J.W. Schwank: A review on TiO2-based nanotubes synthesized via hydrothermal method: Formation mechanism, structure modification, and photocatalytic applications. Catal. Today 225, 34 (2014).
C.J. Dalmaschio and E.R. Leite: Detachment induced by Rayleigh-instability in metal oxide nanorods: Insights from TiO2. Cryst. Growth Des. 12, 3668 (2012).
A.M. Alotaibi, S. Sathasivam, B.A. Williamson, A. Kafizas, C. Sotelo-Vazquez, A. Taylor, D.O. Scanlon, and I.P. Parkin: Chemical vapor deposition of photocatalytically active pure brookite TiO2 thin films. Chem. Mater. 30, 1353 (2018).
A.K. Haridas, B. Gangaja, P. Srikrishnarka, G.E. Unni, A.S. Nair, S.V. Nair, and D. Santhanagopalan: Spray pyrolysis-deposited nanoengineered TiO2 thick films for ultra-high areal and volumetric capacity lithium ion battery applications. J. Power Sources 345, 50 (2017).
A.C. Martins, A.L. Cazetta, O. Pezoti, J.R. Souza, T. Zhang, E.J. Pilau, T. Asefa, and V.C. Almeida: Sol-gel synthesis of new TiO2/activated carbon photocatalyst and its application for degradation of tetracycline. Ceram. Int. 43, 4411 (2017).
D. Nunes, A. Pimentel, L. Santos, P. Barquinha, L. Pereira, E. Fortunato, and R. Martins: Chapter 2—Synthesis, Design, and Morphology of Metal Oxide Nanostructures (Elsevier, Amsterdam, Netherlands, 2019); pp. 21–57.
M. Zimmermann, B. Temel, and G. Garnweitner: Parameter studies of the synthesis of titanium dioxide nanoparticles: Effect on particle formation and size. Chem. Eng. Process. 74, 83 (2013).
M.S. Santos, J.C. Freitas, and C.J. Dalmaschio: Designed single-phase ZrO2 nanocrystals obtained by solvothermal syntheses. CrystEngComm 22, 1802 (2020).
E. Scopel, P.P. Conti, D.G. Stroppa, and C.J. Dalmaschio: Synthesis of functionalized magnetite nanoparticles using only oleic acid and iron (III) acetylacetonate. SN Appl. Sci. 1, 147 (2019).
C.-S. Kim, B.K. Moon, J.-H. Park, S.T. Chung, and S.-M. Son: Synthesis of nanocrystalline TiO2 in toluene by a solvothermal route. J. Cryst. Growth 254, 405 (2003).
M. Niederberger and N. Pinna: Metal Oxide Nanoparticles in Organic Solvents: Synthesis, Formation, Assembly and Application (Springer Science & Business Media, London, United Kingdom, 2009).
G. Demazeau and A. Largeteau: Hydrothermal/solvothermal crystal growth: An old but adaptable process. Z. Anorg. Allg. Chem. 641, 159 (2015).
Z. Fan, F. Meng, M. Zhang, Z. Wu, Z. Sun, and A. Li: Solvothermal synthesis of hierarchical TiO2 nanostructures with tunable morphology and enhanced photocatalytic activity. Appl. Surf. Sci. 360, 298 (2016).
L. Eckertová: Mechanism of Film Formation, in Physics of Thin Films (Springer, New York City, USA, 1977), p. 72.
K.L. Chopra and I. Kaur: Thin Film Phenomena (McGraw-hill, New York, 1969).
R. Ortega-Borges and D. Lincot: Mechanism of chemical bath deposition of cadmium sulfide thin films in the ammonia-thiourea system: In situ kinetic study and modelization. J. Electrochem. Soc. 140, 3464 (1993).
A. Chatterjee, P. Mitra, and A.K. Mukhopadhyay: Chemically deposited zinc oxide thin film gas sensor. J. Mater. Sci. 34, 4225 (1999).
S.S. Lin, A. Hsieh, D.B. Min, and S.S. Chang: A study of the color stability of commercial oleic acid. J. Am. Oil Chem. Soc. 53, 157 (1976).
C. Jia, T. Dong, M. Li, P. Wang, and P. Yang: Preparation of anatase/rutile TiO2/SnO2 hollow heterostructures for gas sensor. J. Alloys Compd. 769, 521 (2018).
Y. Wang, T. Wu, Y. Zhou, C. Meng, W. Zhu, and L. Liu: TiO2-based nanoheterostructures for promoting gas sensitivity performance: Designs, developments, and prospects. Sensors 17, 1971 (2017).
B.L. Cushing, V.L. Kolesnichenko, and C.J. O'Connor: Recent advances in the liquid-phase syntheses of Inorganic nanoparticles. Chem. Rev. 104, 3893 (2004).
H. Tang, K. Prasad, R. Sanjinès, P.E. Schmid, and F. Lévy: Electrical and optical properties of TiO2 anatase thin films. J. Appl. Phys. 75, 2042 (1994).
A. Hastir, N. Kohli, O.S. Kang, and R.C. Singh: Selective liquefied petroleum gas sensor based on nanocomposites of zinc chromium oxide. J. Electroceram. 37, 170 (2016).
L. Hou, C. Zhang, L. Li, C. Du, X. Li, X.-F. Kang, and W. Chen: CO gas sensors based on p-type CuO nanotubes and CuO nanocubes: Morphology and surface structure effects on the sensing performance. Talanta 188, 41 (2018).
N. Chen, Y. Li, D. Deng, X. Liu, X. Xing, X. Xiao, and Y. Wang: Acetone sensing performances based on nanoporous TiO2 synthesized by a facile hydrothermal method. Sens. Actuat., B 238, 491 (2017).
D. Mardare, N. Iftimie, and D. Luca: TiO2 thin films as sensing gas materials. J. Non-Cryst. Solids 354, 4396 (2008).
S. Wang, L. Pan, J.-J. Song, W. Mi, J.-J. Zou, L. Wang, and X. Zhang: Titanium-defected undoped anatase TiO2 with p-type conductivity, room-temperature ferromagnetism, and remarkable photocatalytic performance. J. Am. Chem. Soc. 137, 2975 (2015).
F. Sánchez, U. Lüders, G. Herranz, I. Infante, J. Fontcuberta, M. García-Cuenca, C. Ferrater, and M. Varela: Self-organization in complex oxide thin films: From 2D to 0D nanostructures of SrRuO3 and CoCr2O4. Nanotechnology 16, S190 (2005).
C.J. Dalmaschio, E.G. da Silveira Firmiano, A.N. Pinheiro, D.G. Sobrinho, A.F. de Moura, and E.R. Leite: Nanocrystals self-assembled in superlattices directed by the solvent–organic capping interaction. Nanoscale 5, 5602 (2013).
Acknowledgments
The authors thank the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Fundação de Amparo à Pesquisa e Inovação do Espírito Santo (Fapes), and Federal University of Espírito Santo (Ufes) for the research funding and the scholarships.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dalmaschio, C.J., Conti, P.P., Scopel, E. et al. Nanostructure morphology influences in electrical properties of titanium dioxide thin films. Journal of Materials Research 35, 3012–3020 (2020). https://doi.org/10.1557/jmr.2020.235
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/jmr.2020.235