Abstract
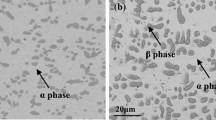
Cr is one of common alloying elements that improve the oxidation resistance of Cu. Its content and distribution are the two important factors that influence the oxidation resistance of alloys. In this paper, the cluster structure model of stable solid solutions was used for designing Cu–Cu12–[Crx/(12+x)Ni12/(12+x)]5 (x = 1, 2, 4, 6 or 8) alloys. The insoluble antioxidative Cr was uniformly distributed in the Cu matrix with the help of Ni element. The solid solution and precipitation of alloys were effectively controlled by changing the Cr/Ni ratio, and the effects of the distribution of alloying elements on the oxidation resistance of Cu alloy were discussed. The studies on isothermal oxidation at 850 °C show that element Cr of the Cu–Ni–Cr alloys designed using cluster model can be dispersed in the Cu matrix, and a continuous protective Cr-rich oxide layer can be formed when the Cr content dispersed in the alloys reaches 9.26 at% during isothermal oxidation. 1/2 of Cr in the Cu–Ni–Cr alloys is replaced by Fe, forming Cu–Ni–(Cr + Fe) alloys; oxide precipitates exhibit a columnar structure revealing the orientation of growth perpendicular to the matrix, thereby forming a lot of O diffusion channels and resulting in a sharp decline in its oxidation resistance. Therefore, the growth morphology of the oxide precipitates may significantly affect the oxidation resistance.







Similar content being viewed by others
References
Y.F. Zhu, K. Mimura, S.H. Hong, and M. Isshik: Influence of small amounts of impurities on initial oxidation of copper at 400 and 800°C in 0.1 MPa oxygen atmosphere. J. Electrochem. Soc. 152, B296 (2005).
G.Y. Fu and Y. Niu: Oxidation behavior of Cu–Cr alloys prepared by different techniques. Acta Metall. Sin. 39, 297 (2009).
Y. Niu, F. Gesmundo, F. Viani, and D.L. Douglass: The air oxidation of two-phase Cu–Cr alloys at 700–900 °C. Oxid. Met. 48, 357 (1997).
C. Wagner: Theoretical analysis of the diffusion processes determining the oxidation rate of alloys. J. Electrochem. Soc. 99, 369 (1952).
F. Gesmundo and Y. Niu: The criteria for the transitions between the various oxidation modes of binary solid-solution alloys forming immiscible oxides at high oxidant pressures. Oxid. Met. 50, 1 (1998).
Z.Q. Cao, Y. Niu, and W.T. Wu: Effect of grain size on the oxidation behavior of Cu–20Ni–20Cr alloy. Rare Met. Mater. Eng. 32, 1016 (2003).
X.J. Zhang, Y. Niu, and W.T. Wu: Cu–20Ni–30Cr oxidation behavior of Cu–20Ni–30Cr alloy in pure O2 at 700°C and 800°C. Rare Met. Mater. Eng. 34, 1271 (2005).
J.X. Chen, Q. Wang, Y.M. Wang, J.B. Qiang, and C. Dong: Cluster formulae for alloy phases. Philos. Mag. Lett. 90, 683 (2010).
C. Dong, Q. Wang, J.B. Qiang, Y.M. Wang, N. Jiang, G. Han, Y.H. Li, J. Wu, and J.H. Xia: From clusters to phase diagrams: Composition rules of quasicrystals and bulk metallic glasses. J. Phys. D: Appl. Phys. 40, R273 (2007).
C. Dong, W.R. Chen, Y.M. Wang, J.B. Qiang, Q. Wang, Y. Lei, M. Calvo-Dahlborg, and J-M. Dubois: Formation of quasicrystals and metallic glasses in relation to icosahe-dral clusters. J. Non-Cryst. Solids 353, 3405 (2007).
J. Zhang, Q. Wang, Y.M. Wang, and C. Dong: Study on the cluster-based model of Ni30Cu70 solid solution with Fe and Mn addition and its corrosion resistance. Acta Metall. Sin. 45, 1390 (2009).
X.N. Li, L.J. Liu, X.Y. Zhang, J.P. Chu, Q. Wang, and C. Dong: Barrierless Cu–Ni–Mo interconnect films with high thermal stability against silicide formation. J. Electron. Mater. 41, 3447 (2012).
X.N. Li, L.R. Zhao, Z. Li, L.J. Liu, C.M. Bao, J.P. Chu, and C. Dong: Barrierless Cu–Ni–Nb thin films on silicon with high thermal stability and low electrical resistivity. J. Mater. Res. 28, 3367 (2013).
X.N. Li, M. Wang, L.R. Zhao, C.M. Bao, J.P. Chu, and C. Dong: Thermal stability of barrierless Cu–Ni–Sn films. Appl. Surf. Sci. 297, 89 (2014).
A. Takeuchi and A. Inoue: Classification of bulk metallic glasses by atomic size difference, heat of mixing and period of constituent elements and its application to characterization of the main alloying element. Mater. Trans. 46 (12), 2817 (2005).
R. Haugsrud and K.L. Lee: On the oxidation behaviour of a Cu–10 vol% Cr in situ composite. Mater. Sci. Eng., A 396, 87 (2005).
Z.Q. Cao, Y. Shen, W.H. Liu, and Y. Xue: Oxidation of two three-phase Cu–30Ni–Cr alloys at 700–800 °C in 1 atm of pure oxygen. Mater. Sci. Eng., A 425, 138 (2006).
Z.Q. Cao, Y. Niu, and G. Farne: Oxidation of the three-phase alloy Cu–20Ni–20Cr at 973–1073 K in 101kpa O2. High Temp. Mater. Processes 20, 377 (2001).
Z.Q. Cao, F. Gesmundo, and M. Al-Omary: Oxidation of a three-phase Cu–45Ni–30Cr alloy at 700–800 °C under 1 atm O2. Oxid. Met. 57, 395 (2002).
Z.Q. Cao and Y. Niu: Oxidation of two ternary Cu–Ni–20Cr alloys at 973–1073 K in 1.01×10−5 kPa O2. High Temp. Mater. Processes 25, 390 (2006).
ACKNOWLEDGMENTS
This project was supported by the National Natural Science Foundation of China (Grant No. 51271045) and the Natural Science Foundation of Jiangsu Province, China (Grant No. BK20131138).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zheng, Y., Li, X., Jin, L. et al. Effects of distribution and growth orientation of precipitates on oxidation resistance of Cu–Cu12–[Crx/(12+x)Ni12/(12+x)]5 alloys. Journal of Materials Research 30, 3299–3306 (2015). https://doi.org/10.1557/jmr.2015.280
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/jmr.2015.280