Abstract
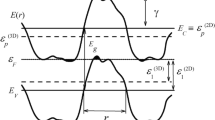
Erbium dihydride Er(H,D,T)2 is a fluorite structure rare-earth dihydride useful for the storage of hydrogen isotopes in the solid state. However, thermodynamic predictions indicate that erbium oxide formation will proceed readily during processing, which may detrimentally contaminate Er(H,D,T)2 films. In this work, transmission electron microscopy (TEM) techniques including energy-dispersive x-ray spectroscopy, energy-filtered TEM, selected area electron diffraction, and high-resolution TEM are used to examine the manifestation of oxygen contamination in ErD2 thin films. An oxide layer ∼30–130 nm thick was found on top of the underlying ErD2 film, and showed a cube-on-cube epitaxial orientation to the underlying ErD2. Electron diffraction confirmed the oxide layer to be Er2O3. While the majority of the film was observed to have the expected fluorite structure for ErD2, secondary diffraction spots suggested the possibility of either nanoscale oxide inclusions or hydrogen ordering. In situ heating experiments combined with electron diffraction ruled out the possibility of hydrogen ordering, so epitaxial oxide nanoinclusions within the ErD2 matrix are hypothesized. TEM techniques were applied to examine this oxide nanoinclusion hypothesis.
Similar content being viewed by others
References
B. Sakintuna, F. Lamari-Darkrim, and M. Hirscher: Metal hydride materials for solid hydrogen storage: A review. Int. J. Hydro-gen Enerev 32, 1121 (2007).
F.E. Lynch: Metal hydride practical applications. J. Less-Common Met. 172–174, 943 (1991).
G.W. Crabtree and M.S. Dresselhaus: The hydrogen fuel alternative. MRS Bull. 33, 421 (2008).
W. Grochala and P.P. Edwards: Thermal decomposition of the non-interstitial hydrides for the storage and production of hydrogen. Chem. Rev. 104, 1283 (2004).
P. Chen and M. Zhu: Recent progress in hydrogen storage. Mater. Today 11, 36 (2008).
J.L. Provo: Effects of vacuum processing erbium dideuteride-ditritide films deposited on chromium underlays on copper substrates. J. Vac. Sci. Technol. 16, 230 (1979).
P.A. Dow, G.W. Briers, M.A.P. Dewey, and D.S. Stark: Structure of erbium deuteride targets for neutron generators. Nucl. Instrum. Methods 60, 293 (1968).
H.T. Bach, F.J. Steinkruger, W.S. Chamberlin, and C.R. Walthers: Quantitative analysis of deuterium and tritium in erbium hydride films of neutron tube targets. J. Vac. Sci. Technol., B 22. 1738 (2004).
D.L. Chichester and J.D. Simpson: Compact accelerator neutron generators. Industr. Physicist 9, 22 (2003–2004).
I. Gabis, E. Evard, A. Voyt, I. Chernov, and Y. Zaika: Kinetics of decomposition of erbium hydride. J. Alloys Compd. 356, 353 (2003).
E.J. Fernandez and D.M. Holloway: Oxidation studies of erbium hydride system. J. Vac. Sci. Technol. 11, 612 (1974).
D.J. Mitchell and R.C. Patrick: Temperature dependence of helium release from erbium tritide films. J. Vac. Sci. Technol. 19, 236 (1981).
C.R. Tewell and S.H. King: Observation of metastable erbium trihydride. Appl. Surf. Sci. 253, 2597 (2006).
A.E. Curzon and H.G. Chlebek: Observation of face centered cubic erbium in thin-films and its oxidation. J. Less-Common Met. 27, 411 (1972).
M.S. Rahman Khan: Epitaxial growth of erbium dihydride films. Thin Solid Films 113, 207 (1984).
M.S. Rahman Khan and R.F. Miller: The growth and structure of epitaxial films of the rare-earth dihydrides. J. Phys. D: Appl. Phys. 12, 271 (1979).
R.S. Blewer and J.K. Maurin: Dimensional expansion and surface microstructure in helium-implanted erbium and erbium-hydride films. J. Nucl. Mater. 44, 260 (1972).
J.W. Guthrie, L.C. Beavis, D.R. Begeal, and W.G. Perkins: Properties of hydride-forming metals and of multilayer hydrogen permeation barriers. J. Nucl. Mater. 53, 313 (1974).
E.D. Gu, H. Savaloni, M.A. Player, and G.V. Marr: Characterization of evaporated erbium films at various stages of growth. J. Phys. Chem. Solids 53, 127 (1992).
M.S. Rahman Khan: Changes produced in the electrical resistivity of ErH2 thin films when converted to ErH3 due to hydrogen treatment. Appl. Phys. A 35, 263 (1984).
J.A. Knapp and J.F. Browning: Nanoindentation characterization of ErT2 thin films. J. Nucl. Mater. 350, 147 (2006).
C.S. Snow, L.N. Brewer, D.S. Gelles, M.A. Rodriguez, P.G. Kotula, M.A. Mangan, and J.F. Browning: Helium release and microstructural changes in Er(D,T)2–x3Hex films. J. Nucl. Mater. 374, 147 (2007).
P. Vajda: Hydrogen ordering and metal-semiconductor transitions in superstoichiometric rare earth dihydrides. J. Alloys Compd. 231, 170 (1995).
R.T. DeHoff: Thermodynamics in Materials Science (McGraw-Hill, New York, 1993).
D.M. Holloway: The quantitative determination of surface oxide thickness on deposited metal films by combination auger spectroscopy and inert gas ion bombardment. Appl. Spectrosc. 27, 95 (1973).
D.F. Cowgill: Helium nano-bubble evolution in aging metal tritides. Fus. Sci. Technol. 48, 539 (2005).
E. Fromm and H. Uchida: Effect of oxygen sorption layers on the kinetics of hydrogen absorption by tantalum at 77–700 K. J. Less-Common Met. 66, 77 (1979).
H. Wenzl, K-H. Klatt, P. Meuffels, and K. Papathanassopoulos: Hydrogen storage in thin film metal hydrides. J. Less-Common Met. 89, 489 (1983).
I.P. Jain, B. Devi, and A. Williamson: Hydrogen in UHV deposited FeTi thin films. Int. J. Hydrogen Energy 26, 1183 (2001).
T. Venhaus and J. Poths: Observations on He-3 release from ErT2 films. Fus. Sci. Technol. 48, 601 (2005).
R. Brydson: Electron Energy Loss Spectroscopy (Royal Microscopical Society Handbook #48) (BIOS Scientific Publishers, Oxford, 2001).
G. Kothleitner and F. Hofer: Optimization of the signal to noise ratio in EFTEM elemental maps with regard to different ioniza-tion edge types. Micron 29, 349 (1998).
J. Mayer: Nanoscale analysis by energy-filtering TEM, in Advances in Imaging and Electron Physics, Vol. 123, edited by P.W. Hawkes (Academic Press, Amsterdam, 2002), p. 399.
P.J. Thomas and P.A. Midgley: An introduction to energy-filtered transmission electron microscopy. Top. Catal. 21, 109 (2002).
D.B. Williams and C.B. Carter: Transmission Electron Microscopy (Plenum, New York, 1996).
I.P. Jain, Y.K. Vijay, L.K. Malhotra, and K.S. Uppadhyay: Hydrogen storage in thin-film metal hydride-A review. Int. J. Hydrogen Energy 13, 15 (1988).
E.J. Grier, A.K. Petford-Long, and R.C.C. Ward: Determination of hydrogen ordering within the ss-RH2 + x phase (R = Ho, Y) using electron diffraction techniques. J. Appl. Crystallogr. 33, 1246 (2000).
J.A. Goldstone, J. Eckert, P.M. Richards, and E.L. Venturini: Temperature and concentration-dependence of hydrogen site occupancy in several rare-earth dihydrides. Phys. B + C (Amsterdam) 136, 183 (1986).
P. Vajda, J.N. Daou, and J.P. Burger: Observations of magnetic and structural ordering in TbH2+x compounds through electrical-resistivity measurements. Phys. Rev. B 36, 8669 (1987).
S.N. Sun, Y. Wang, and M.Y. Chou: First principles study of hydrogen ordering in b-YH2+x. Phys. Rev. B 49, 6481 (1994).
T.J. Udovic, J.J. Rush, and I.S. Anderson: Neutron spectroscopic evidence of concentration-dependent hydrogen ordering in the octahedral sublattice of b-TbH2+x. Phys. Rev. B 50, 7144 (1994).
P. Vajda and J.N. Daou: Magnetic and metal-semiconductor transitions in ordered and disordered ErH(D)(2+x). Phys. Rev. B 49, 3275 (1994).
T.J. Udovic, J.J. Rush, and I.S. Anderson: Neutron spectroscopic comparison of b-phase rare-earth hydrides. J. Alloys Compd. 231, 138 (1995).
I.G. Ratishvili and P. Vajda: Hydrogen ordering in superstoichiometric rare-earth hydrides for a system with an energy-constants ratio p = V2/V1 < 1: LaH2+x. Phys. Rev. B 53, 581 (1996).
T.J. Udovic, Q. Huang, and J.J. Rush: Hydrogen and deuterium site separation in fcc-based mixed-isotope rare-earth hydrides. Phys. Rev. B 61, 6611 (2000).
M. De Graef: Introduction to Conventional Transmission Electron Microscopy (Cambridge University Press, Cambridge, 2003).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Parish, C.M., Snow, C.S. & Brewer, L.N. The manifestation of oxygen contamination in ErD2 thin films. Journal of Materials Research 24, 1868–1879 (2009). https://doi.org/10.1557/jmr.2009.0217
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/jmr.2009.0217