Abstract
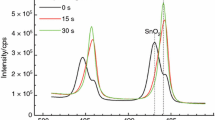
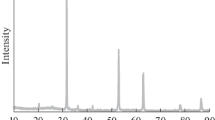
Differential scanning calorimetry experiments on mixed Cu2-xS, ZnS, and SnS2 precursors were conducted to better understand how Cu2ZnSnS4 (CZTS) and Cu2SnS3 form. The onset temperatures of Cu2SnS3 reactions and CZTS suggest that the ZnS phase may mediate Cu2SnS3 formation at lower temperatures before a final CZTS phase forms. We also found no evidence of a stable Cu2ZnSn3S8 phase. The major diffraction peaks associated with Cu2ZnSnS4, and Cu2SnS3 (overlaps with ZnS, as well) began to grow around 380 °C, although the final reaction to form Cu2ZnSnS4 probably did not occur until higher temperatures were reached. An exothermic reaction was observed corresponding to formation of this phase. There was some variability in the onset temperature for reactions to form Cu2SnS3. At least 5 steps are involved in this reaction and several segments of the reaction had relatively reproducible energies.
Similar content being viewed by others
References
W. Wang, M.T. Winkler, O. Gunawan, T. Gokmen, T.K. Todorov, Y. Zhu, and D.B. Mitzi, Adv. Energy Mater.4, 1301465 (2014).
T. Maeda, S. Nakamura, and T. Wada, Jpn. J. Appl. Phys.50, 04DP07 (2011)
R. Schurr, A. Hölzing, S. Jost, R. Hock, T. Voβ, J. Schulze, A. Kirbs, A. Ennaoui, M. Lux-Steiner, A. Weber, I. Kötschau, and H.-W. Schock, Thin Solid Films517, 2465 (2009)
A. Fairbrother, X. Fontané, V. Izquierdo-Roca, M. Espíndola-Rodríguez, S. López-Marino, M. Placidi, L. Calvo-Barrio, A. Pérez-Rodríguez, and E. Saucedo, Sol. Energy Mater. Sol. Cells112, 97 (2013)
A. Weber, R. Mainz, T. Unold, S. Schorr, and H.-W. Schock, Phys. Status Solidi6, 1245 (2009)
S. Schorr, A. Weber, V. Honkimäki, and H.W. Schock, Thin Solid Films517, 2461 (2009)
S. V. Baryshev and E. Thimsen, Chem. Mater.27, 2294 (2015).
O. Kubaschewski, C.B. Alcock, and P.J. Spencer,Materials Thermochemistry, 6th ed. (Pergamon Press, New York, 1993) pp. 258–323
M.J. Buerger and B.J. Wuensch, Science (80-. ).141, 276 (1963).
M.Y. Valakh, V.M. Dzhagan, I.S. Babichuk, X. Fontane, A. Perez-Rodriquez, and S. Schorr, JETP Lett.98, 255 (2013).
M. Dimitrievska, A. Fairbrother, A. Pérez-Rodríguez, E. Saucedo, and V. Izquierdo-Roca, Acta Mater.70, 272 (2014).
M. Paris, L. Choubrac, A. Lafond, C. Guillot-Deudon, and S. Jobic, Inorg. Chem.53, 8646 (2014).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Pogue, E.A., Goetter, M. & Rockett, A. Reaction kinetics of Cu2-xS, ZnS, and SnS2 to form Cu2ZnSnS4 and Cu2SnS3 studied using differential scanning calorimetry. MRS Advances 2, 3181–3186 (2017). https://doi.org/10.1557/adv.2017.384
Published:
Issue Date:
DOI: https://doi.org/10.1557/adv.2017.384