Abstract
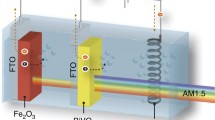
Given the limitations of the materials available for photoelectrochemical water splitting, a multiphoton (tandem) approach is required to convert solar energy into hydrogen efficiently and durably. Here we investigate a promising system consisting of a hematite photoanode in combination with dye-sensitized solar cells with newly developed organic dyes, such as the squaraine dye, which permit new configurations of this tandem system. Three configurations were investigated: two side-by-side dye cells behind a semitransparent hematite photoanode, two semitransparent dye sensitized solar cells (DSCs) in front of the hematite, and a trilevel hematite/DSC/DSC architecture. Based on the current-voltage curves of state-of-the-art devices made in our laboratories, we found the trilevel tandem architecture (hematite/SQ1 dye/N749 dye) produces the highest operating current density and thus the highest expected solar-to-hydrogen efficiency (1.36% compared with 1.16% with the standard back DSC case and 0.76% for the front DSC case). Further investigation into the wavelength-dependent quantum efficiency of each component revealed that in each case photons lost as a result of scattering and reflection reduce the performance from the expected 3.3% based on the nanostructured hematite photoanodes. We further suggest avenues for the improvement of each configuration from both the DSC and the photoanode parts.
Similar content being viewed by others
References
A. Fujishima, K. Honda Electrochemical photolysis of water at a semiconductor electrode. Nature 238, 37 (1972)
O. Khaselev, J.A. Turner A monolithic photovoltaic-photoelectrochemical device for hydrogen production via water splitting. Science 280, 425 (1998)
B.D. Alexander, P.J. Kulesza, L. Rutkowska, R. Solarska, J. Augustynski Metal oxide photoanodes for solar hydrogen production. J. Mater. Chem. 18, 2298 (2008)
van de R. Krol, Y.Q. Liang, J. Schoonman Solar hydrogen production with nanostructured metal oxides. J. Mater. Chem. 18, 2311 (2008)
M. Gratzel Photoelectrochemical cells. Nature 414, 338 (2001)
M.F. Weber, M.J. Dignam Efficiency of splitting water with semiconducting photoelectrodes. J. Electrochem. Soc. 131, 1258 (1984)
A.B. Murphy, P.R.F. Barnes, L.K. Randeniya, I.C. Plumb, I.E. Grey, M.D. Horne, J.A. Glasscock Efficiency of solar water splitting using semiconductor electrodes. Int. J. Hydrogen Energy 31, 1999 (2006)
A. Kay, I. Cesar, M. Gratzel New benchmark for water photooxidation by nanostructured alpha-Fe2O3 films. J. Am. Chem. Soc. 128, 15714 (2006)
J. Augustynski, G. Calzaferri, J.C. Courvoisier, M. Gratzel Photoelectrochemical hydrogen production: State of the art with special reference to IEA’s hydrogen programme 11th World Hydrogen Energy Conference (11 WHEC) edited by T.N. Veziroglu, C.J. Winter, J.P. Baselt, and G. Kreysa (Dechema, Stuttgart, Germany 1996) 2379
A. Duret, M. Gratzel Visible light-induced water oxidation on mesoscopic a-Fe2O3 films made by ultrasonic spray pyrolysis. J. Phys. Chem. B 109, 17184 (2005)
M.K. Nazeeruddin, P. Pechy, T. Renouard, S.M. Zakeeruddin, Humphry-R. Baker, P. Comte, P. Liska, L. Cevey, E. Costa, V. Shklover, L. Spiccia, G.B. Deacon, C.A. Bignozzi, M. Gratzel Engineering of efficient panchromatic sensitizers for nanocrystalline TiO2-based solar cells. J. Am. Chem. Soc. 123, 1613 (2001)
H. Arakawa, C. Shiraishi, M. Tatemoto, H. Kishida, D. Usui, A. Suma, A. Takamisawa, T. Yamaguchi Solar hydrogen production by tandem cell system composed of metal oxide semiconductor film photoelectrode and dye-sensitized solar cell. Solar Hydrogen and Nanotechnology II 65003, 65003 (2007)
J.H. Yum, P. Walter, S. Huber, D. Rentsch, T. Geiger, F. Nuesch, De F. Angelis, M. Gratzel, M.K. Nazeeruddin Efficient far red sensitization of nanocrystalline TiO2 films by an unsymmetrical squaraine dye. J. Am. Chem. Soc. 129, 10320 (2007)
T. Bessho, E. Yoneda, J-H Yum, M. Guglielmi, I. Tavernelli, H. Imai, U. Rothlisberger, M.K. Nazeeruddin, M. Gratzel New paradigm in molecular engineering of sensitizers for solar cell applications. J. Am. Chem. Soc. 131, 5930 (2009)
Standard tables for reference solar spectral irradiances Direct normal and hemispherical on 37° tilted surface. G 173-03 Annual Book of ASTM Standards (ASTM International, West Conshohocken, PA 2003)
L.M. Peter Dye-sensitized nanocrystalline solar cells. Phys. Chem. Chem. Phys. 9, 2630 (2007)
S. Ito, S.M. Zakeeruddin, P. Comte, P. Liska, D.B. Kuang, M. Gratzel Bifacial dye-sensitized solar cells based on an ionic liquid electrolyte. Nat. Photonics 2, 693 (2008)
A. Mihi, M.E. Calvo, J.A. Anta, H. Miguez Spectral response of opal-based dye-sensitized solar cells. J. Phys. Chem. C 112, 13 (2008)
A. Devos Detailed balance limit of the efficiency of tandem solar-cells. J. Phys. D 13, 839 (1980)
M.K. Nazeeruddin, Humphry-R. Baker, P. Liska, M. Gratzel Investigation of sensitizer adsorption and the influence of protons on current and voltage of a dye-sensitized nanocrystalline TiO2 solar cell. J. Phys. Chem. B 107, 8981 (2003)
M. Durr, A. Bamedi, A. Yasuda, G. Nelles Tandem dye-sensitized solar cell for improved power conversion efficiencies. Appl. Phys. Lett. 84, 3397 (2004)
I. Cesar, K. Sivula, A. Kay, R. Zboril, M. Gratzel Influence of feature size, film thickness, and silicon doping on the performance of nanostructured hematite photoanodes for solar water splitting. J. Phys. Chem. C 113, 772 (2009)
Y-S Hu, Kleiman-A. Shwarsctein, G.D. Stucky, E.W. McFarland Improved photoelectrochemical performance of Ti-doped a-Fe2O3 thin films by surface modification with fluoride. Chem. Commun. 19, 2652 (2009)
D.K. Zhong, J. Sun, H. Inumaru, D.R. Gamelin Solar water oxidation by composite catalyst/a-Fe2O3 photoanodes. J. Am. Chem. Soc. 131, 6086 (2009)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Brillet, J., Cornuz, M., Formal, F.L. et al. Examining architectures of photoanode–photovoltaic tandem cells for solar water splitting. Journal of Materials Research 25, 17–24 (2010). https://doi.org/10.1557/JMR.2010.0009
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/JMR.2010.0009