Abstract
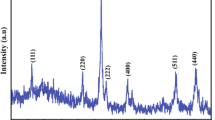
Nanosized particles of γ–Fe2O3 were prepared by heat treatment of the precipitates, obtained from a homogeneous solution of stearic acid and hydrated iron(III) nitrate. The compositional and thermal characteristics of the precipitates were studied with the aid of infrared spectroscopy and differential scanning calorimetry. The x-ray diffraction and small-angle x-ray scattering investigation shows that γ–Fe2O3 nanoparticles with narrow size distribution can be prepared successfully by this route.
Similar content being viewed by others
References
M. P. Morales, M. Andres-Verges, S. Veintemillas-Verdaguer, M.I .Montero, and C. J. Serna, J. Magn. Magn. Mater. 203,146 (1999).
J. L. Dormann, P. Deb, and A. Basumallick, J. Magn. Magn. Mater. 203, 23 (1999).
S. Grimm, M. Schultz, S. Barth, and R. Muller, J. Mater. Sci. 32, 1083 (1997).
R. P. Cowbern, A. M. Moulinand, and M. E. Welland, Appl. Phys. Lett. 71, 2202 (1997).
T. Ishikawa, T. Takeda, and K. Kandori, J. Mater. Sci. 27, 4531 (1992).
O. V. Lounansmaa, Phys. Scr. 66, 70 (1996).
S. C. Davies, C. Ni, J. Fardyu, and D. Rassi, J. Med. Eng. Technol. 18, 127 (1994).
O. Glatter and O. Kratky, Small Angle X-ray Scattering (Academic Press, London, United Kingdom, 1982).
A. Guiner, G. Fournet, B. C. Walker, and L.K. Yudowith Small Angle Scattering of X-Rays (Wiley, New York, 1955).
M. K. Wilson, in Determination of organic structures by physical methods, edited by F.C. Nachod and W.D. Phillips (Academic Press, New York, 1962), Vol. 2, Chapter 3, p. 181.
J.R. Ryder Application of Absorption Spectroscopy of Organic Compounds (Prentice Hall of India, New Delhi, India, 1987), p.22.
A. Miller, Arzeinmitel Forsch. 17, 921 (1967).
M. P. Morales, C. Percharroman, T. Gonzales Carreno, and C.J. Serna, J. Solid State Chem. 108, 158 (1994).
N. Irving Sax and R. J. Lewis, Sr., Hawley’s Condensed Chemical Dictionary, 11th ed. (CBS publication and distributors, New Delhi, India, 1990), p. 1091.
S. Enzo, G. Fagherazzi, A. Benedetti, and S. Polizzi, J. Appl. Crystallogr. 21, 536 (1988).
Th. H. DE Keijser, E. Mittemeijer, and H.C. F. Rozenda, J. Appl. Crystallogr. 16, 309 (1983).
D. Sen, P. Deb, S. Mazumder, and A. Basumallick, Mater. Res. Bull. 35, 1243 (2000).
C.S. Hwang, J. Mater . Sci. Eng. B 56, 178 (1998).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Deb, P., Basumallick, A. Preparation of γ–Fe2O3 nanoparticles from a nonaqueous precursor. Journal of Materials Research 16, 3471–3475 (2001). https://doi.org/10.1557/JMR.2001.0477
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/JMR.2001.0477